An End-To-End Automated HTP Platform For Cell Line Optimization
By Whitney Liu, Ph.D., Bristol Myers Squibb

CHO cells remain the workhorse for biotherapeutics production due to their ability to produce complex proteins with proper folding, assembly, and human-compatible post-translational modifications. However, conventional CHO cell line development (CLD) workflows are slow, low-throughput, and may require repeated vector reformatting — bottlenecks that hinder rapid discovery and development timelines. In an era where pipeline diversity is expanding to include more complex biologics such as bispecific antibodies and novel scaffolds, there is an increasing need for faster, scalable, and versatile production platforms.
At Bristol Myers Squibb, we have developed an automated high-throughput (HTP) stable CHO platform leveraging Leap-In transposase technology, integrated automation, and engineered host cell lines (Figure 1). This system produces high-titer, high-quality material in a fraction of the conventional timeline, supporting everything from early-stage screening to large-scale production.
Figure 1
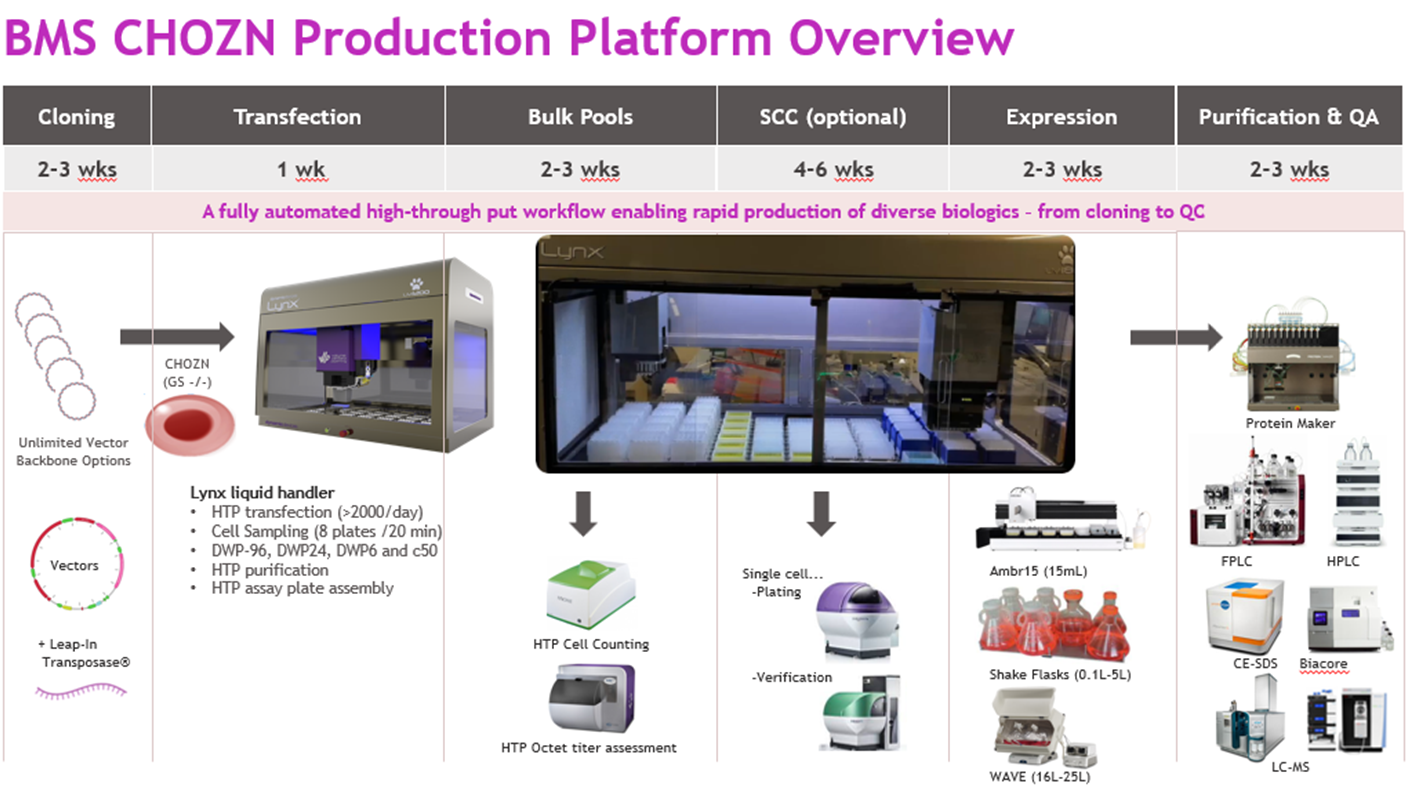
Source: Whitney Liu
The platform can handle >1,000 stable pool transfections in parallel, uses dual-antibiotic selection to improve bispecific yield and fidelity, supports vector optimization for productivity gains up to 6 g/L, and incorporates the CHOplus engineered host to boost titers by three and a half times. In practice, this means moving from DNA to large-scale fed-batch production in seven to 10 weeks, cutting about a month off the standard process.
This insight is particularly valuable for organizations looking to accelerate hit-to-lead progression and enable parallelized development for complex biologics without compromising product quality.
The Problem: Traditional Platforms Have Intrinsic Bottlenecks
Historically, the CHOZN platform has been the basis for stable biologics expression at BMS. While robust and well characterized, it involves a sequential and labor-intensive process — often taking over three months from DNA to large-scale material. For high-throughput discovery programs, this creates major bottlenecks, especially when producing large antibody panels or screening multiple bispecific formats.
The introduction of Leap-In transposase technology provided an opportunity to transform CLD efficiency. Unlike random integration, Leap-In supports multiple site-specific integrations, producing highly stable pools in shorter timelines (Figure 2). By pairing this with integrated automation (Lynx, NyOne, Octet, Zephyr, VIPS, Ambr15) and optimized selection strategies, we created a true end-to-end HTP platform.
Figure 2
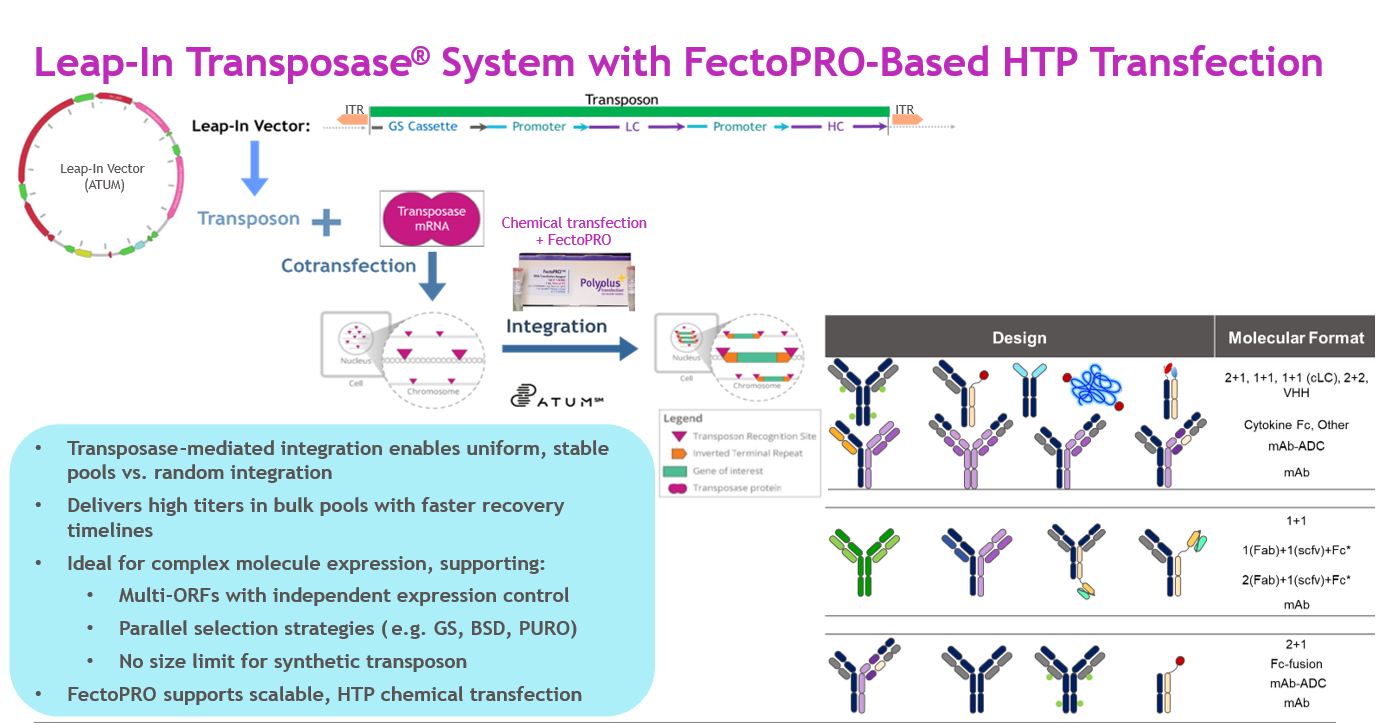
Source: Whitney Liu
This approach allows early material supply directly from recovering stable pools, eliminating the need for separate transient expression campaigns. For complex molecules like bispecifics, the dual-antibiotic selection approach minimizes non-productive cell drift, maintaining both yield and fidelity. Recent advances with CHOplus, an engineered CHOZN host enriched for endoplasmic reticulum (ER) capacity, have further improved titers and recovery speed.
Together, these innovations address long-standing CLD pain points: long timelines, low throughput, and variable yields for complex biologics.
The Solution: Case Studies In Platform Production
Our automated HTP CHO platform is designed to support end-to-end stable expression — from DNA to fed-batch material — while integrating automation, data tracking, and flexible scaling. Four representative case studies illustrate its versatility and impact.
Case Study 1: High-throughput mAb expression at scale
In one discovery program, we were tasked with screening 575 monoclonal antibodies (mAbs) and delivering large-scale material within a highly compressed timeline (Figure 3). Using the HTP platform, we co-transfected >1,000 stable pools in CHOZN cells (dual-vector format, duplicates) via Leap-In transposase. Selection and recovery were monitored on the Lynx automated workstation, NyOne imaging system, and Octet titer analysis.
Figure 3
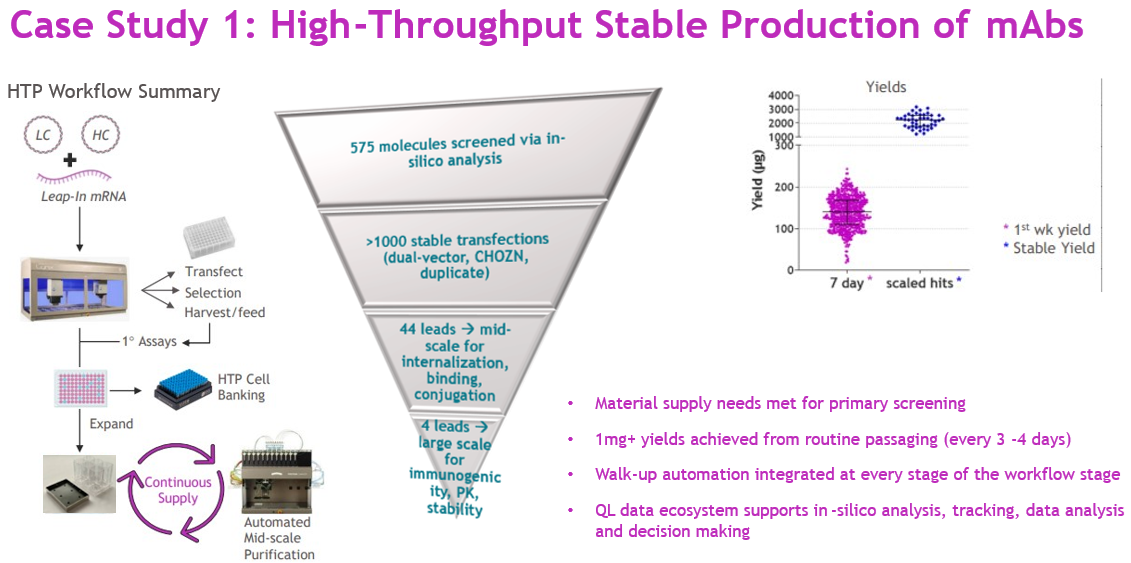
Source: Whitney Liu
By day seven post-transfection, small-scale material (~150 μg) from recovering pools was available for primary screening — meeting assay requirements without waiting for full recovery. Once pools recovered (day 14), we froze working stocks and could continuously supply 1 mg to 2 mg from routine passages every three to four days. The final four lead mAbs advanced to large-scale fed-batch production for immunogenicity, PK, and stability testing.
This approach shortened the timeline by approximately two months compared to a traditional process, which would have required separate transient campaigns and sequential stable transfections (Figure 4). The efficiency allowed the entire workflow — from >1,000 pools to large-scale production — to be executed by a single full-time employee.
Figure 4
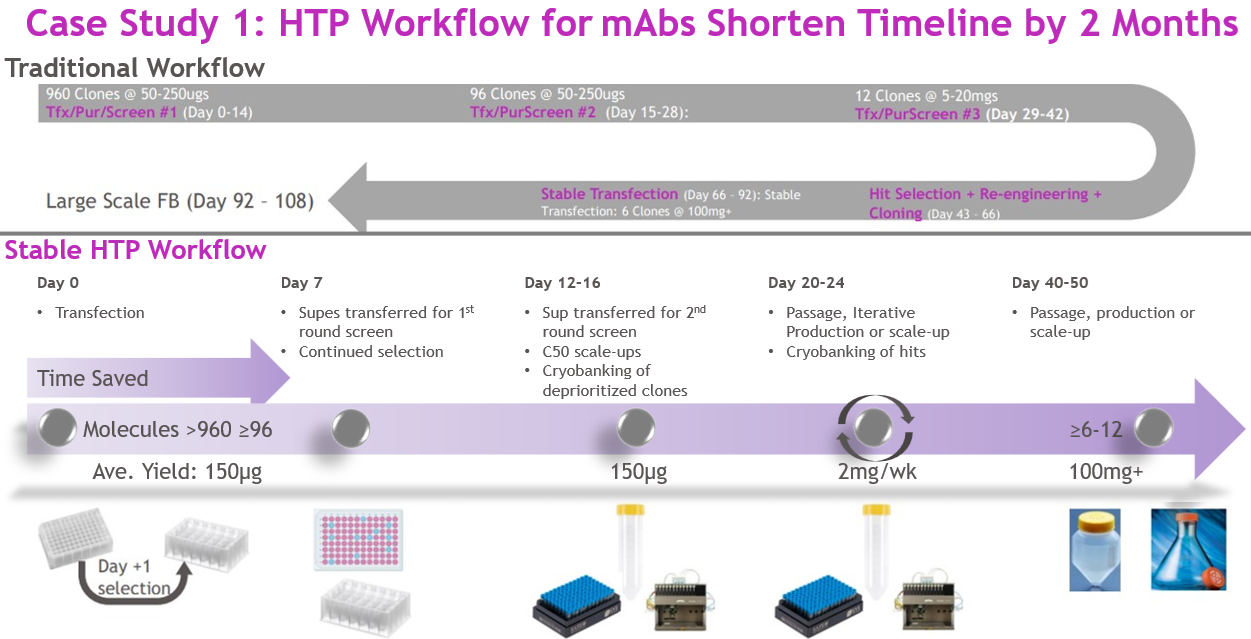
Source: Whitney Liu
Case Study 2: Improving bispecific expression via dual selection
Bispecific antibodies often suffer from production drift, where non-productive clones dominate during selection, lowering yield and product fidelity. We developed a dual-antibiotic selection strategy to address this (Figure 5).
Vectors were designed with heavy chain 1 (HC1) and heavy chain 2 (HC2) each flanked by different antibiotic resistance markers — either blasticidin (BSD) or puromycin (Puro). Based on killing curve assessments in CHOZN cells, BSD and Puro provided faster recovery compared to hygromycin or neomycin.
Figure 5
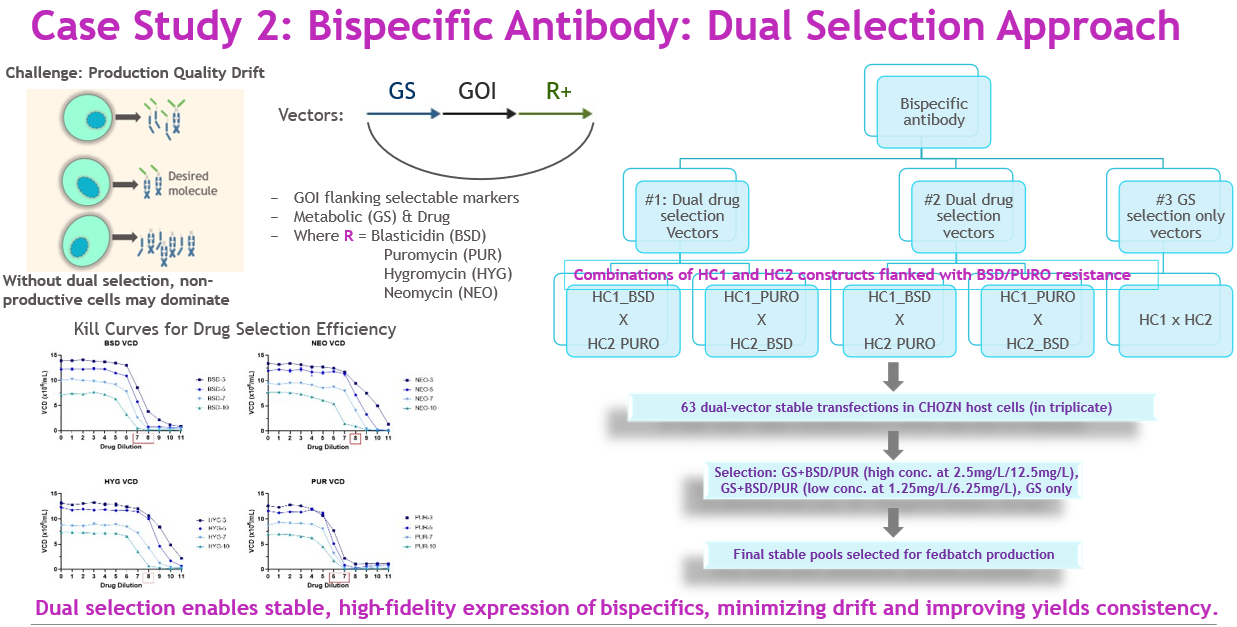
Source: Whitney Liu
Testing two backbone designs, we performed 63 stable transfections in triplicate under three conditions: GS + BSD/Puro (high), GS + BSD/Puro (low), and GS only. The highest-yielding pool reached 2.7 g/L — over three times higher than the GS-only control — using the high-concentration dual selection (Figure 6). This strategy has since been incorporated into our standard HTP workflow for multi-specific formats, delivering consistent yield and quality across projects.
Figure 6
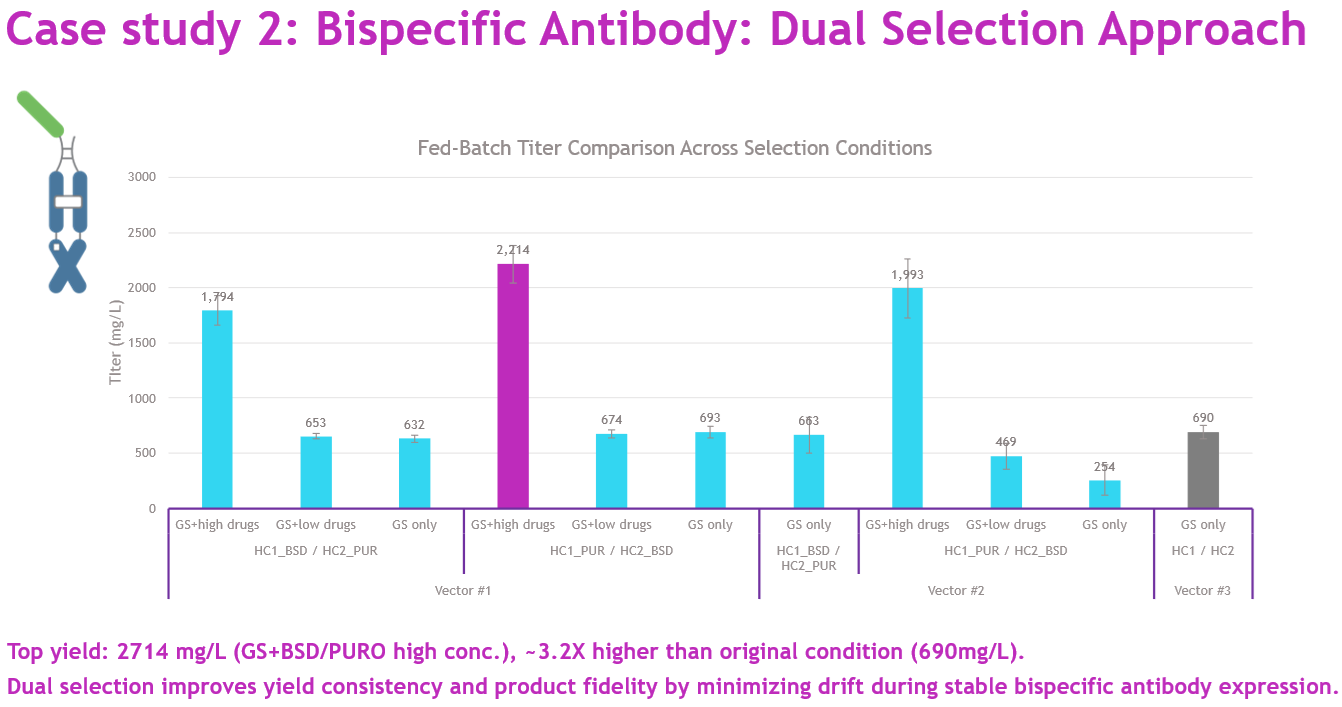
Source: Whitney Liu
Case Study 3: Vector optimization for yield improvement
For a challenging mAb program, we screened 10 backbone vectors, two promoters, and two signal peptides, evaluating 60 stable transfections under six different selection conditions (GS, GS+Puro, GS+BSD, GS+Neo, GS+Hyg, BSD only) (Figure 7).
Figure 7
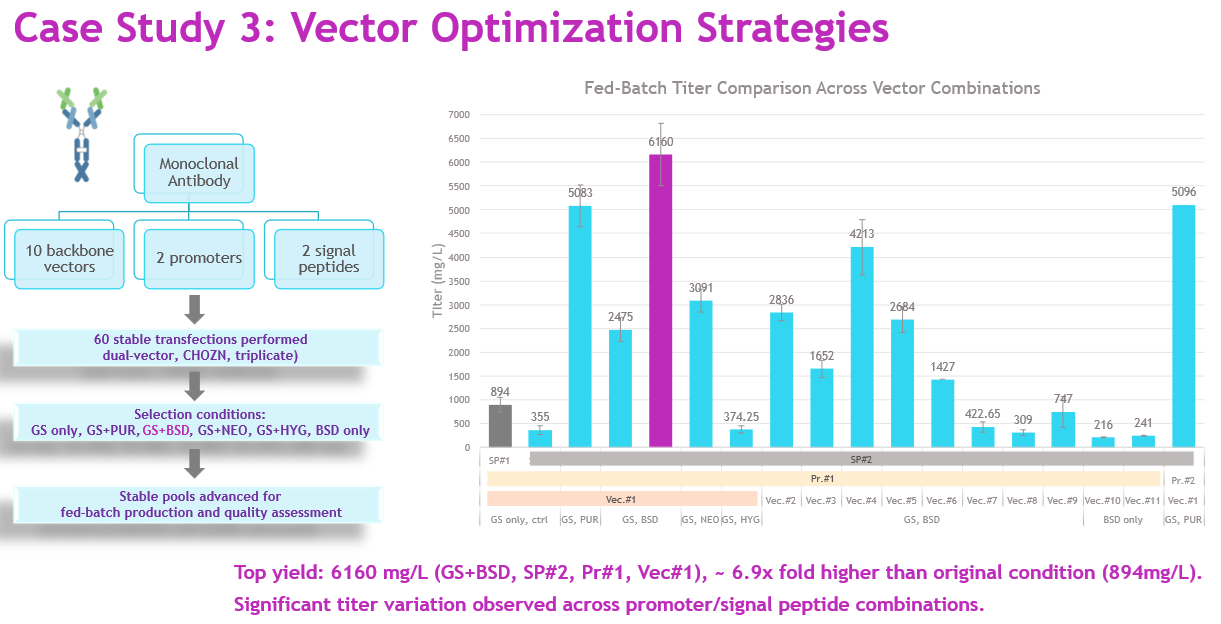
Source: Whitney Liu
The best-performing combination — backbone vector #1 with promoter #1, signal peptide #2, and GS+BSD selection — produced >6 g/L titer, a nearly sevenfold improvement over the baseline construct. This demonstrates how the HTP platform enables extensive vector exploration within a single campaign, providing rapid, data-driven optimization of expression systems.
Case Study 4: CHOplus engineered host for higher productivity
To further boost capacity, we partnered with CHOplus to engineer a CHOZN host with enhanced ER capacity. After multiple rounds of genome engineering and cloning, two CHOplus clones were selected and integrated into our HTP workflow (Figure 8).
Figure 8
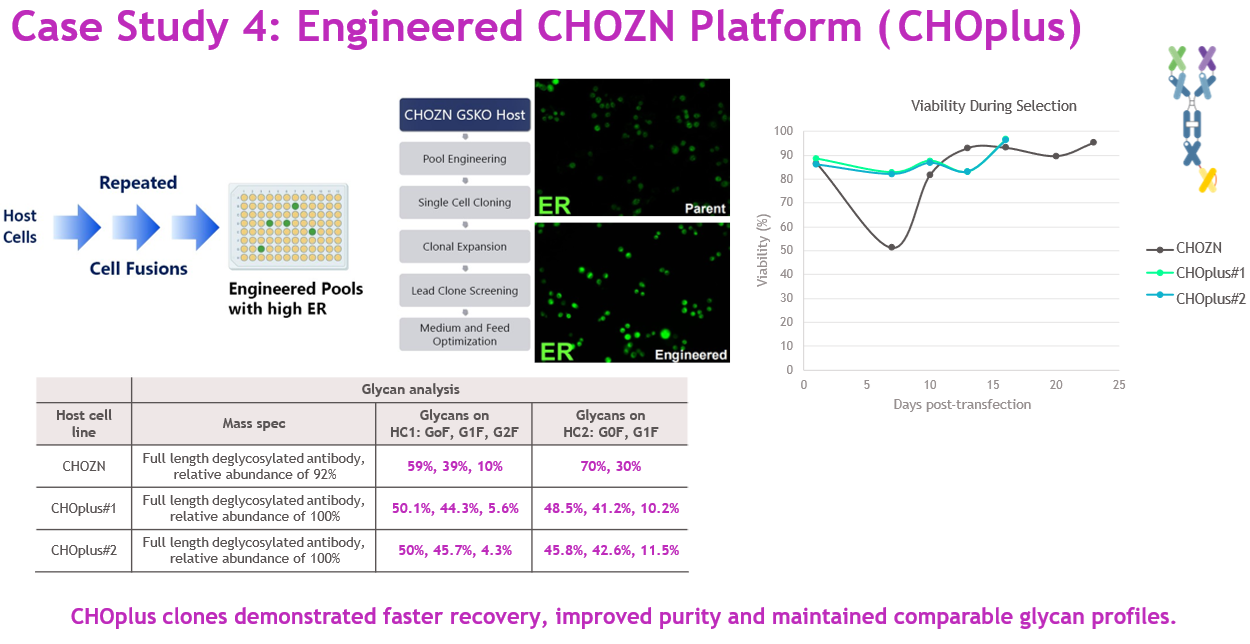
Source: Whitney Liu
In a bispecific program, pools in both CHOplus hosts recovered one week faster than pools in CHOZN and produced higher titers. Large-scale fed-batch (5 L) results showed (Figure 9):
- stable pools in CHOZN: harvested day 7 due to low viability; 330 mg/L average titer
- stable pools in CHOplus #1: harvested days 12 to 15; 1.14–1.18 g/L titer
- stable pools in CHOplus #2: harvested day 12; 690 mg/L titer.
Figure 9
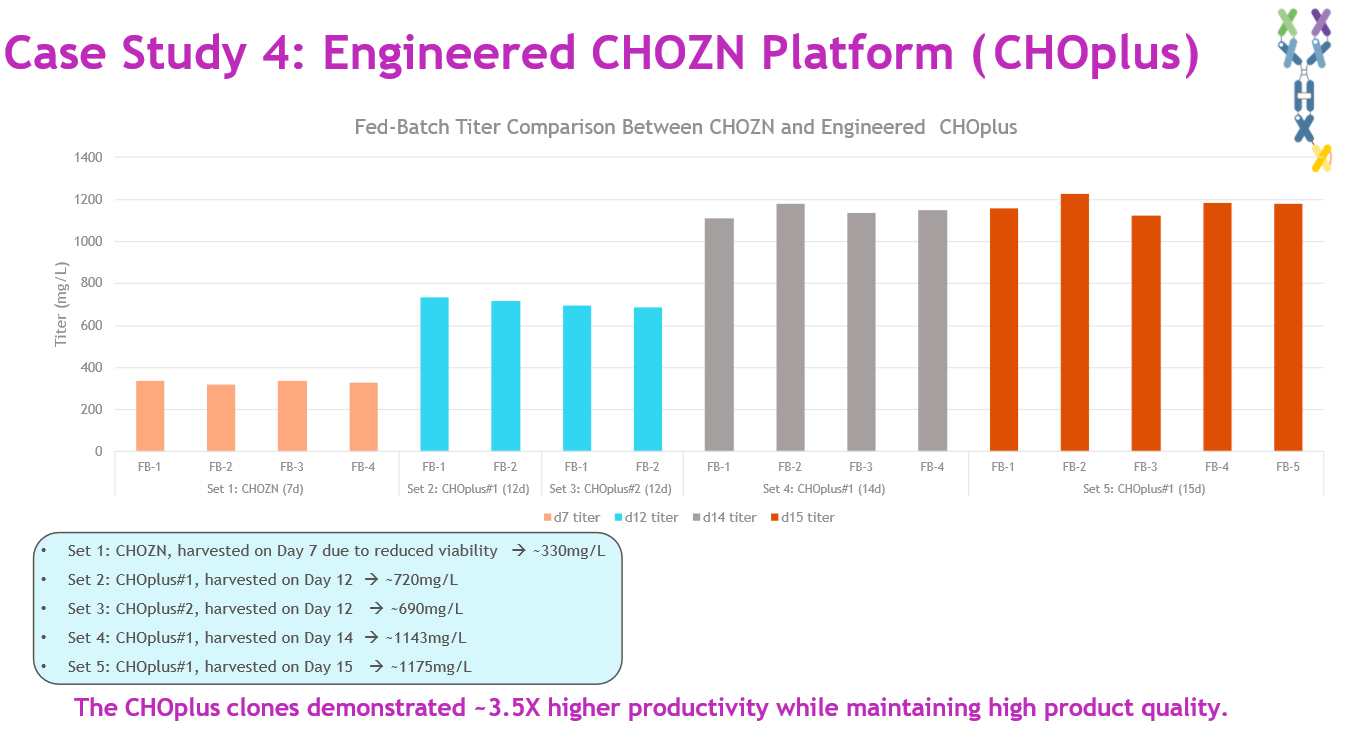
Source: Whitney Liu
Using CHOplus #1 delivered a three and a half-fold productivity increase while maintaining comparable purity and glycan profiles, validating the benefit of host engineering for complex biologics.
Collectively, these case studies demonstrate that the BMS HTP stable CHO platform is not just a speed enabler — it is a comprehensive, flexible, and scalable solution that can handle diverse molecule formats, integrate optimization, and deliver high-quality material from microgram to gram scale within compressed timelines.
The Platform Has Few Barriers, But They’re Worth Noting
While the HTP platform offers clear speed and scalability benefits, adoption may be constrained by equipment costs, integration complexity, and training needs. Leap-In licensing and material transfer agreements may also be required. For very low-expressing or unstable constructs, additional optimization cycles may still be necessary.
Conclusion
The automated HTP stable CHO platform fundamentally changes how we approach early-stage biologics production, offering speed, throughput, and flexibility without sacrificing quality. For discovery teams facing complex, multi-format pipelines, this approach accelerates timelines, expands screening capacity, and enables earlier decision-making.
Editor’s Note: The author wishes to acknowledge contributors to this work, with special thanks to Michelle Vendel, SJ Diong, Tommy Su, Mariko Riley, Cristian Alberto, Ashby Fenby-Lenoir, Lindsey O’Heron, Lawrence Dearth, Henry Chan, and Payal Sheth.
About the Author:
 Whitney Liu, Ph.D., is the principal scientist in discovery biotherapeutics and lead discovery and optimization at Bristol Myers Squibb, where she leads high-throughput automated protein production platform development, with expertise with expertise in cell line development, automation integration, and expression process optimization. She earned her Ph.D. in microbiology from Nankai University in China, followed by five years of postdoctoral training at the University of California, San Diego, and The Scripps Research Institute in La Jolla, gaining extensive academic expertise in protein sciences. Following her postdoctoral work, Liu transitioned into the biopharmaceutical industry, where she has spent the past 10 years advancing biotherapeutics. Connect with her on LinkedIn.
Whitney Liu, Ph.D., is the principal scientist in discovery biotherapeutics and lead discovery and optimization at Bristol Myers Squibb, where she leads high-throughput automated protein production platform development, with expertise with expertise in cell line development, automation integration, and expression process optimization. She earned her Ph.D. in microbiology from Nankai University in China, followed by five years of postdoctoral training at the University of California, San Diego, and The Scripps Research Institute in La Jolla, gaining extensive academic expertise in protein sciences. Following her postdoctoral work, Liu transitioned into the biopharmaceutical industry, where she has spent the past 10 years advancing biotherapeutics. Connect with her on LinkedIn.
