Biologics CMO

With patients’ lives at the end of the pipeline, biologics contract manufacturing needs to be safe, reliable and consistent in supply. The developers of these large molecule drugs, however, are also operating in a highly competitive space. Speed-to-market is imperative, along with efficient processes and low costs. A lot of this will rest on the quality of the cell line development and the expertise of the biologics CMO you choose.
AbbVie Contract Manufacturing has a proven track record in biopharmaceutical process development and is committed to treating your project as if it were our own. We understand the importance of strong partnerships with our customers and the need for confidence in our team. AbbVie maintains one of the most trusted reputations in the biologics CMO industry and is known for reliability, flexibility, and scientific expertise.
BIOLOGICS CONTRACT MANUFACTURING
AbbVie Contract Manufacturing offers exceptional end-to-end biologics capabilities with some of the world’s most advanced production facilities. As a leading biologics CMO, we offer the following expertise and more:
- Cell line development
- Process optimization
- Fermentation biotechnology
- Analytical characterization
- cGMP manufacturing
- Validation
CELL CULTURE MANUFACTURING
Success in biologics contract manufacturing is largely measured by product reproducibility. Through sophisticated cell line development, cell culture manufacturing and quality control, a biologics CMO must overcome the complexity of large molecule manufacturing to deliver consistent drug performance.
AbbVie Contract Manufacturing excels at biopharmaceutical process development, establishing the groundwork for a sustainable, reliable biologics program and ongoing drug supply.
LARGE MOLECULE DRUGS – ADDITIONAL CAPABILITIES
- Process development, optimization and scale up
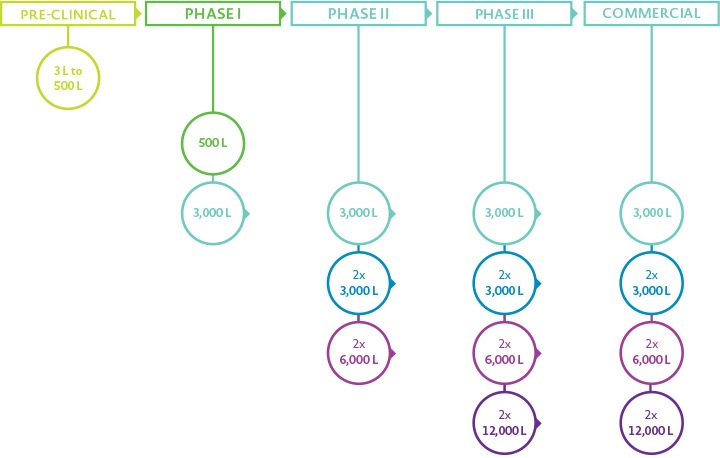
- cGMP manufacturing form 500 L – 12,000 L
- Global marketing approval and regulatory expertise
- Structured project management support and analytical services
- Exceptional reputation for milestone execution and reliability
ANALYTICAL SERVICES – ADDITIONAL CAPABILITIES
-
HPLC (SEC, IEX, affinity, reverse phase)
- Host-cell protein
- Electrophoresis (CE, IEF, SDS-PAGE)
- ELISA
- Peptide structure determination (LC, MS)
- Oligosaccharide analysis
- DNA quantification (threshold)
- DNA identification (PCR)
- Spectrophotometry (FT-IR, UV VIS)
- Bioburden / endotoxin
CLIENT SERVICES – ADDITIONAL CAPABILITIES
-
Stability studies
- Regulatory filing expertise
- Skilled planning and project management
- Scale to accomodate multiple customers simultaneously
- Dedicated time slots for biologics contract manufacturing
- Exceptional track reacord of meeting all timelines
