Biologics Analytical Development
Source: Alcami

Leveraging robust analytical services at every stage of development and manufacturing is crucial to ensure your therapy reaches patients in need on time and within budget.
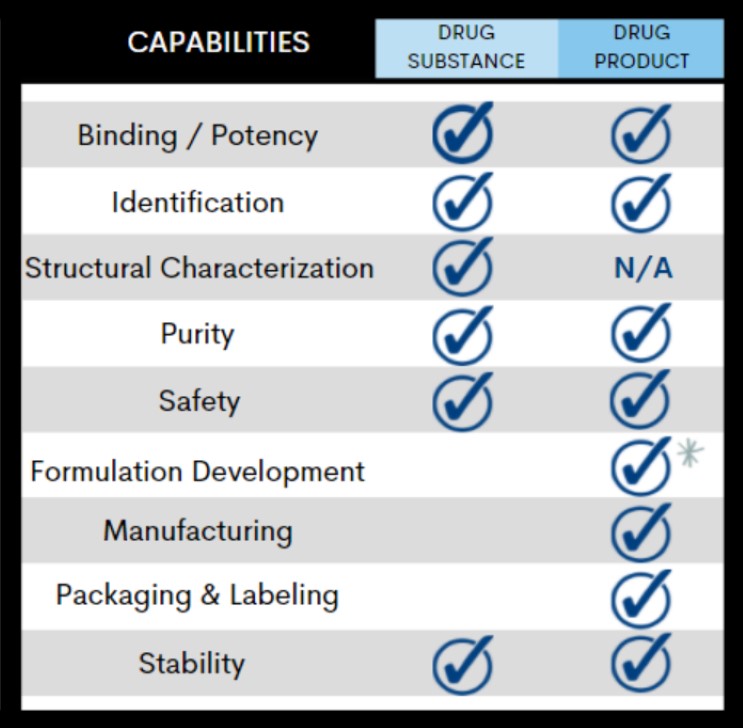
From initial characterization of drug substance to commercial batch release, each phase of your drug product development requires analytical methods to qualify the product for safety, integrity, strength, purity, and quality.
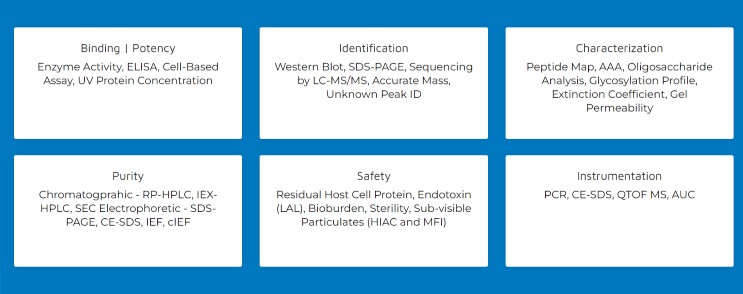
With these critical attributes in mind, Alcami’s biologics analytical development team will work with you to design a robust control strategy to support every phase of your product development lifecycle:
- Dedicated formulations chemists
- Phase appropriate analytical development and testing
- Aseptic manufacturing, fill-finish, and lyophilization capabilities
- Extensive structural characterization services
- Comprehensive ICH compliant stability services
- Drug substance and drug product stability indicating assay
- Related substances, chiral purity, elemental impurity, and residual solvent analysis
- Dissolution testing with UV and HPLC backend
- Preservative and stabilizing excipients assays
- Phospholipid and fatty acid analysis
- Physical and structural chemistry
- Remedial method validation/method lifecycle evaluation studies
- Drug substance and reference standard material characterization
- Reference standard qualification
- Process validation support
- Cleaning method verification
- Drug product comparator studies
- Material contact studies
- In-use and administration set compatibility studies
- Extractables and leachables studies