A Brief Overview Of Vaccine Purification Technologies And Analytical Tools
By Tony D’Amore and Yan-ping Yang

Vaccination is the most powerful and cost-effective way to protect billions of people around the world and has made an enormous contribution to global health. According to WHO, immunization awareness and government initiatives have helped prevent 2 million to 3 million deaths annually.14 Based on country-reported data, WHO estimates that, across 47 vaccines and 94 manufacturers, the 2021 global vaccine market supplied approximately 16 billion vaccine doses (up from 5.8 billion in 2019), including COVID vaccines, with a value of $141 billion (up from $38 billion in 2019). This value accounts for 10% of the pharmaceutical market (up from 4% in 2019).23
The methods used for viral and bacterial protein sub-unit vaccine purification were described previously.26 The technology of vaccine development has improved significantly over the years, from the earliest vaccines developed as live-attenuated or inactivated pathogens to the engineering of new strains and new systems based on plants or insect larvae that emerged as alternative, low-cost platforms.6 To develop a vaccine candidate against the emerging COVID-19 pandemic, two new types of platforms, mRNA-based and viral-vector-based vaccines, have been the first to reach the market due to the rapid advancement in their methodologies.5, 20, 24
Downstream Purifications: Challenges And Opportunities
Each vaccine has its unique production process. From bacterial-based vaccine manufacturing to the production of viral-based vaccines (Figure 1), downstream processing is the most complex and demanding aspect in vaccine manufacturing.13, 15, 27, 28
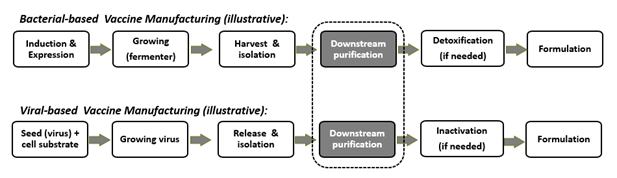
Figure 1: Schematic presentation of vaccine manufacturing
As shown in Figure 2A, downstream processing is aimed at eliminating contaminants originating from host cells or culture media and producing large volumes of concentrated biologically active ingredient (i.e., purified bulk) with the implementation of multiple unit operations, such as column chromatography, ultrafiltration (UF), and diafiltration (DF), etc. (Figure 2B)
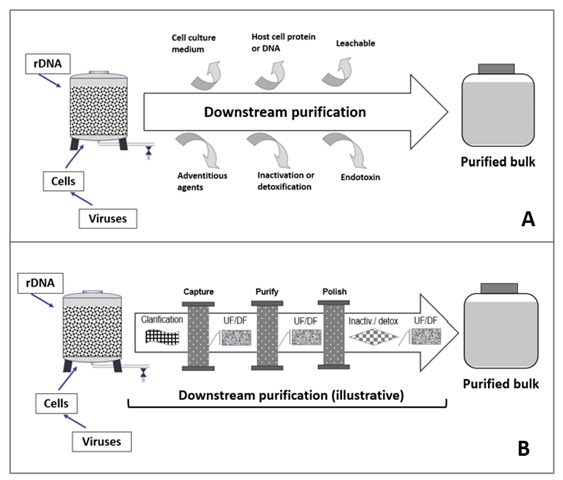
Figure 2: Schematic presentation of downstream purification
There is clearly a need and a desire for change and innovation in the vaccine industry. This is driven by increased market demand, product shortage, increased regulatory requirements, the push for product reproducibility, simplification, and harmonization of processes, and to meet the demand for the changing infectious disease landscape. The industry is under constant pressure to generate a return on investment while getting to clinic as soon as possible. For legacy products, there is also a pressure to ensure product quality and supply while overcoming challenges presented by aging facilities, equipment, and legacy processes.
Advancement Of Purification Technologies And Analytics
As illustrated in Figure 3, downstream technology advancement is driven by a number of factors, such as:
- Technology advancements in neighboring industries that inspire new technology development for bioprocessing.
- Improvements in sensitivities of analytical tools.
- Innovative ideas from the academic world.
- Pressure from improved expression levels.
- Demand for higher production yields.
- Increased regulatory requirements to remove impurities.
These demands have created great opportunities for vendors and labs to come up with innovative solutions to address downstream challenges.2, 10 Over the years, suppliers and vaccine manufacturers have collectively made great efforts to develop new technologies and solutions for bioprocessing, such as the co-development of the sterile packed bed chromatography system3 and a disposable and fully closed system for the purification of non-sterilizable virus-based vaccines.25
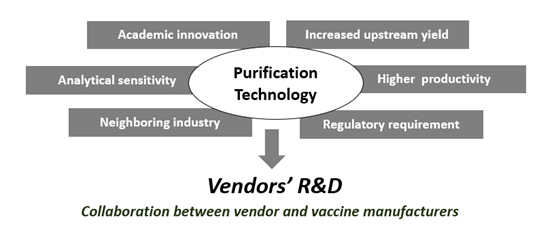
Figure 3: Main drivers for purification technology development
Implementation Of New Purification Technologies In Vaccine Process Development
The evolution and breakthroughs in purification technologies and analytical tools over the last three decades have benefited the vaccine industry. For example, the availability of high-throughput process development and analytical tools has accelerated vaccine process development and contributed to the reduction of process development cycle time,27, 28 which leads to faster clinical proof of concept and shorter time to market. The introduction of single-use disposable technology into bioprocessing has simplified the establishment of new production lines and facilities and increased vaccine process development and manufacturing flexibility. The emerging concept and application of continuous manufacturing in vaccine production will further help to reduce the cost of goods and capital expenditure and, at the same time, ensure consistent product quality due to steady state operation.8
The significant time saving from using high-throughput liquid handling systems for purification development was reported earlier.12, 27, 28 It was demonstrated that, while it took about two months to complete the 24 resin screening studies under the conventional approach, only eight days were required with the application of a high-throughput liquid handling system. In addition, application of disposable technologies was found to generate significant financial benefits, such as reduced capital costs of up to 40% compared to traditional hard-piped facilities,16, 18, 19 and financial savings from reduced time to clinic of up to one year.19
Implementation Of State-of-the-Art Analytical Tools To Support Vaccine Development
Development of new vaccines is highly dependent on the availability of analytical methods for their design and evaluation. As shown in Table 2, significant advancements in analytical technology achieved over the last three decades have improved testing paradigms in vaccine development, while also supporting existing vaccine manufacturing and quality control.7
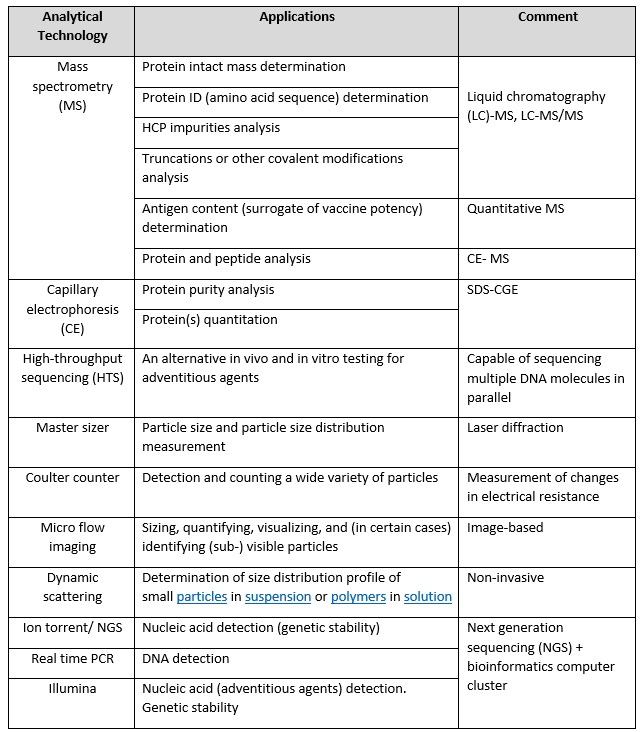
Table 2: Summary of advanced analytical methods used in vaccine development.
The Potential Of mRNA To Deliver New Vaccines
The swift delivery of the world’s first mRNA-based COVID-19 vaccine puts a big spotlight on the promise of mRNA technology.5, 24 As shown in Table 3, unlike the traditional vaccines, mRNA-based vaccines (such as the Pfizer and Moderna COVID-19 vaccines) provide a blueprint for human cells to make a protein that triggers an immune response.21 The manufacturing of mRNA is sequence-independent, which makes it highly adaptable to different pathogens and fast delivery.
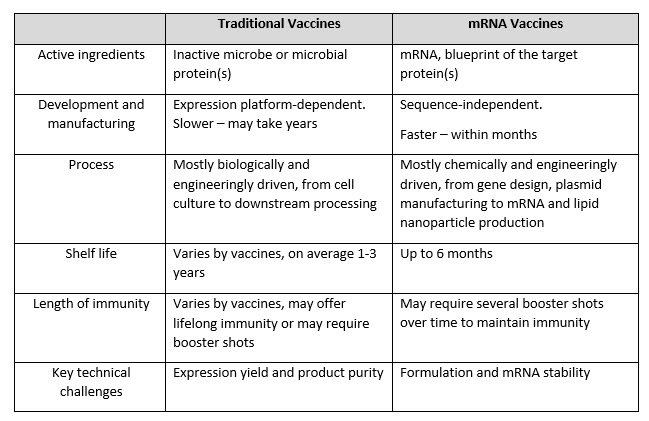
Table 3: Comparison of traditional vaccines and mRNA- based vaccines
One of the most important advantages of mRNA over conventional vaccines is its relatively simple and sequence-independent manufacturing, from upstream processing (comprises the enzymatic generation of mRNA) and downstream processing (includes multi-unit operations required to purify the mRNA product) to lipid nanoparticle (LNP) formulation and fill/finish steps.17
Downstream processing, together with fill/finish, is still the major bottleneck in mRNA vaccine production due to the lack of well-established and cost-effective processes. Despite the effort to develop methods that achieve high-purity products, most of them are coupled with the traditional precipitation or nuclease digestion techniques.1, 22 Moreover, most methods are not cost-effective. Alternative cost-effective techniques, such as single-pass tangential flow filtration (SPTFF) or aqueous two-phase systems (ATPS), that can be applied in a continuous mode, may potentially improve process time and manufacturing flexibility while reducing cost and maintaining quality.9 In addition, new chromatographic operation modes, such as multimodal chromatography, could overcome the need for multiple mRNA purification steps.11
Conclusions
There have been significant advances in purification technology over the years, leading to higher yield and quality products. However, there continues to be pressure on vaccine and biological industries to reduce cost, increase quality and yield, and provide solutions to meet unmet medical needs. This article demonstrates the benefits of innovations in vaccine purification methods and their impact. The work continues to enhance the overall product development process, in particular for downstream purification and the resurgence caused by the introduction of novel methods such as mRNA.
References
- Baiersdörfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U. (2019) A Facile Method for the Removal of dsRNA Contaminant from In Vitro Transcribed mRNA. Mol Ther - Nucleic Acids 2019; 15:26–35.
- Bolton, GR., Violand, BN., Wright, RS., Sun, S., Sunasara, KM., Waltson, K., Coffman, JL., Gallo, C., Godavarti, R. (2011) Addressing the Challenges in Downstream Processing Today and Tomorrow. BioPharm Int Suppl 24 (4): s8-s15.
- Braendli E. & Lemmens R. (2010) Sterile Packed Bed Chromatography System: A Case Study. PDA- Annual Meeting 2010, March 15-19. Orlando, FL
- Charlebois R.L., Ng S.H.S. Gisonni-Lex L., and Mallet L. (2014) Cataloguing the Taxonomic Origins of Sequences from a Heterogeneous Sample using Phylogenomics: Applications in Adventitious Agent Detection. PDA J Pharm Sci and Tech (2014) 68: 602-618
- Chow D. (2020) What is mRNA? How Pfizer and Moderna tapped new tech to make coronavirus vaccines. https://www.nbcnews.com/science/science-news/what-mrna-how-pfizer-moderna-tapped-new-tech-make-coronavirus-n1248054
- Cid R. & Bolívar J. (2021) Platforms for Production of Protein-Based Vaccines: From Classical to Next-Generation Strategies. Biomolecules. 2021 Aug; 11(8): 1072. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8394948/
- D’Amore T. (2019) Accelerating Development through Innovation: Advances & Challenges in Vaccine Development. BPI West 2019, 11 – 14 March. Santa Clara, CA
- D’Amore T. and Yang Y-P. (2013) Increasing Flexibility in Vaccine Manufacturing Processes and Facilities with Single-use Disposable Technologies. BioPharma Asia, July/August 2013. p8-17.
- Fisher A.C., Kamga M-H., Agarabi C., Brorson K., Lee S.L., Yoon S. (2019) The Current Scientific and Regulatory Landscape in Advancing Integrated Continuous Biopharmaceutical Manufacturing. Trends Biotechnol. 2019;37(3):253–67.
- Gottschalk U. (2006) The Renaissance of Protein Purification. BioPharm Int. June suppl p 8-9.
- Halan V., Maity S., Bhambure R., Rathore A.S. (2018) Multimodal Chromatography for Purification of Biotherapeutics - A Review. Curr Protein Pept Sci 2018;20 (1):4–13.
- LI C. (2012) Application of High Throughput Technology in Purification Process Development. 2nd International Conference in High-Throughput Process Development 2012, June 4 – 7. France
- Lightfoot E.N. & Moscariello J.S. (2004) Bioseparations. Biotechnol. Bioeng. 87: 259-273.
- Market Research Report (2022) Fortune Business Insights. https://www.fortunebusinessinsights.com/industry-reports/vaccines-market-101769
- Morenweiser R. (2005) Downstream Processing of Viral Vectors and Vaccines. Gene Therapy. Vol12: pS103–S110
- País-Chanfrau J.M., Chico E., and Zorrilla K. (2009) The Impact of Disposables on Project Economics in a New Antibody Plant: A Case Study. BioPharm International. 2009 (22): 12.
- Rosa S.S.; Prazeres D.M.F., Azevedo A.M., Marques M.P.C. (2021) mRNA Vaccines Manufacturing: Challenges and Bottlenecks. Vaccine 2021 Apr 15; 39(16): 2190–2200.
- Shukla A. and Gottschalk U. (2013) Single-use Disposable Technologies for Biopharmaceutical Manufacturing. Trends Biotechnol. 2013; (3):147-154.
- Sinclair A. & Monge M. (2005) Concept Facility Based on Single-use Systems. BioProcess International. 2005 (3):51-55.
- Ura, T., Yamashita, A., Mizuki, N., Okuda, K., Shimada, M. (2021) New vaccine Production Platforms Used in Developing SARS-CoV-2 Vaccine Candidates. Vaccine 2021, 39, 197–201.
- Vanderbilt Institute (2020) How Does a mRNA Vaccine Compare to a Traditional Vaccine? https://www.vumc.org/viiii/infographics/how-does-mrna-vaccine-compare-traditional-vaccine
- Von Der Mülbe F., Reidel L., Ketterer T., Gontcharova L., Bauer S., Pascolo S., et al. (2015) Method for producing rna. PCT/EP2015/000959; US10017826B2; WO/ 2016/180430A1.
- WHO: Global Vaccine Market Report 2022. https://www.who.int/publications/m/item/global-vaccine-market-report-2022
- Willett J.D.S. 2020. COVID-19 Vaccines: How Pfizer’s and Moderna’s 95% Effective mRNA Shots Work. The Conversation. https://theconversation.com/covid-19-vaccines-how-pfizers-and-modernas-95-effective-mrna-shots-work-149957
- Xiong Y., Braendli E., Yang Y-P. (2010) A Disposable and Fully Closed System for the Purification of Non-Sterilizable Virus. IBC- Biopharmaceutical Development & Production Week 2010, March 1-5. Carlsbad, CA
- Yang Y-P. & D’Amore T. (2014) Protein Subunit Vaccine Purification (Chapter 6), in: Vaccine Development and Manufacturing. Publisher: Wiley (ISBN - 13:9780470261941), p181-216
- Yang Y-P. (2016) Advances in Purification Technologies Accelerate Vaccine Development. American Pharmaceutical Review. July/August 2016, Volume 19, Issue 5, p52-59.
- Yang Y-P. (2017) From Purification to Vaccine Development: A Challenging Yet Rewarding Journey. BioPharma Asia, September/October 2017. p28-34.
About The Authors
 Tony D’Amore, Ph.D., MBA, joined Sanofi Pasteur in 1994 as a purification scientist. He has taken on successive leadership positions over the years in product development and the manufacture of clinical trial material. From July 2015 to January 2022 he was vice-president of product research and development, responsible for process and analytical development, manufacture of clinical material and delivery to clinical trial sites. D’Amore is retired and provides consulting services in the area of CMC for vaccines and biologics. He holds a HBSc and Ph.D. in biochemistry from the University of Windsor and an MBA from Wilfred Laurier University. Reach him by email tony.damore56@gmail.com.
Tony D’Amore, Ph.D., MBA, joined Sanofi Pasteur in 1994 as a purification scientist. He has taken on successive leadership positions over the years in product development and the manufacture of clinical trial material. From July 2015 to January 2022 he was vice-president of product research and development, responsible for process and analytical development, manufacture of clinical material and delivery to clinical trial sites. D’Amore is retired and provides consulting services in the area of CMC for vaccines and biologics. He holds a HBSc and Ph.D. in biochemistry from the University of Windsor and an MBA from Wilfred Laurier University. Reach him by email tony.damore56@gmail.com.
 Yan-ping Yang, Ph.D., joined Sanofi Pasteur in 1989 as a researcher with increasing roles and responsibilities over a career of more than 30 years. From July 2014 to April 2022 at Sanofi, she was associate vice president, head of bioprocess research and development, North America, overseeing the efforts to develop vaccine manufacturing processes to support preclinical and clinical studies up to Phase 3. Yang retired from Sanofi in April 2022 and is now an independent consultant in the area of CMC for vaccines and biologics. She holds a Ph.D. in biochemistry from the University of Missouri-Columbia.
Yan-ping Yang, Ph.D., joined Sanofi Pasteur in 1989 as a researcher with increasing roles and responsibilities over a career of more than 30 years. From July 2014 to April 2022 at Sanofi, she was associate vice president, head of bioprocess research and development, North America, overseeing the efforts to develop vaccine manufacturing processes to support preclinical and clinical studies up to Phase 3. Yang retired from Sanofi in April 2022 and is now an independent consultant in the area of CMC for vaccines and biologics. She holds a Ph.D. in biochemistry from the University of Missouri-Columbia.
