ARTICLES BY RAJENDRAN (RAJ) ARUNAGIRI
-
8/21/2025
The FDA has become more vigilant about alcohol-induced dose dumping in extended-release oral dosages. Such dumping can result in anything from reduced therapeutic effects to dangerous toxicity. Let's take a closer look.
-
4/21/2025
While the effectiveness of GLP-1 in weight loss is good news, the use of organic solvents in the GLP-1 synthesis and purification process is a growing pain that needs to be addressed.
-
11/19/2024
Enteric coatings play a pivotal role for drugs/APIs that need to withstand the harsh conditions of the stomach in order to dissolve in the intestine. This article shares an overview, commonly used excipients, and more.
-
4/18/2024
Titanium dioxide (TiO2) is used as a food additive and OSD pharmaceutical excipient. In 2020, the European Food Safety Authority noted data gaps regarding particle size, which can affect its toxicological properties, so the European Commission banned it as a food additive. Here's how this could impact pharma.
-
3/6/2024
On Feb. 6, 2024, new legislation was introduced in the House of Representatives: the FDA Modernization Act 3.0. It aims to reduce and replace the use of animals in nonclinical research, improve predictivity of nonclinical testing, and potentially reduce drug development times. This article shares an overview. Note that the proposed legislation would still need to go through its course of passing the House and Senate and being signed by the president before becoming a law.
-
2/2/2024
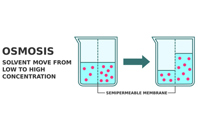
Osmotic controlled release drug delivery is not often spoken about, but it is a useful oral drug formulation technology. This article shares the mechanisms of the elements of oral dosage, including the semi-permeable membrane, pore-forming agents, and more, as well as advantages and applications of this type of drug delivery.
-
12/18/2023
One of the toughest challenges facing small molecule drug development today is poor solubility of the API. No single technique has universal application, so several strategies exist. This article examines four notable strategies.
-
8/3/2023
Speed to formulation plays a critical role in recouping the investment for a new drug. Many ingredient suppliers have an experienced analytical group and material scientist group, and drug developers should reach out and leverage that expertise with these four strategies.
-
6/12/2023
The United States’ reliance on foreign manufacturers of API has been a known fact for several years. We can mitigate our supply vulnerabilities, but bio/pharma companies and the FDA both have roles to fill in this need.
-
5/8/2023
As the world discovers new molecular entities, new solutions for and innovation of excipients are more critical than ever. However, they are often overlooked. Let’s look at how to address three key challenges: risk aversion, an inconsistent feedback loop between pharma companies and excipient suppliers, and the current lack of regulatory body-backed innovation programs.