FEATURED EDITORIAL
Fragmented processes are stalling progress. By aligning CDMOs through transparency and standards, developers can move past the COGS obsession and stabilize the supply chain.
- 2025 GLP-1 Impacts Tell Us About CDMOs In 2026
- Contamination Control Strategies In Low Bioburden Biologic Drug Substance Manufacturing
- What 2025 FDA Warning Letters Tell Us About GMP Compliance
- 2025 Review: Customer Service As Important As Capacity And Capability
- The Module Type Package Wants All Your Equipment To Start Talking
- FDA Issues Final Guidance For pH Adjuster Waiver Requests For Generic Drugs
- Four Reasons You Should Choose Lyophilization for Your Next Project
GUEST COLUMNISTS
-
Contamination Control Strategies In Low Bioburden Biologic Drug Substance Manufacturing
How do manufacturers apply a contamination control strategy in a non-sterile world, particularly at a low bioburden biologic drug substance manufacturing site? How can Annex 1 apply?
-
What 2025 FDA Warning Letters Tell Us About GMP Compliance
This analysis identifies key compliance trends and regional disparities by examining the 2025 U.S. FDA warning letters issued to drug product manufacturers posted between Jan. 1 and Dec. 9, 2025.
-
The Module Type Package Wants All Your Equipment To Start Talking
A forthcoming ISPE guide uses case studies to aid in the design of plug-and-play process skids with implications for every step in the manufacturing process continuum.
-
FDA Issues Final Guidance For pH Adjuster Waiver Requests For Generic Drugs
The FDA has issued a final guidance, Considerations for Waiver Requests for pH Adjusters in Generic Drug Products Intended for Parenteral, Ophthalmic, or Otic Use.
-
Four Reasons You Should Choose Lyophilization for Your Next Project
Lyophilization ensures product stability, extends shelf life, supports sensitive formulations, and simplifies storage and transport, making it a critical choice for modern drug development projects.
-
Why Your MVP And Its Evolution Matters To Manufacturing
Minimum viable product is one of the most important inputs to developing a manufacturing process. It defines essentials that matter to the people who make the product.
-
TFF Offers Ideal Anaerobic, GMP Conditions For LBP Concentration
When configured correctly, TFF provides the gentle processing environment and oxygen protection required for strict anaerobes.
-
Biotech Wasn't Ready For AI's Speed; Here's How We Catch Up
Computational success is no stand-in for therapeutic readiness. Innovators must learn to discern between models rooted in science and those that speculate.
PHARMA OUTSOURCING WHITE PAPERS
-
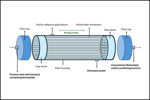
Solving The AAV Scalability Challenge
Explore the effectiveness of a flexible, scalable AAV manufacturing platform that incorporates well-characterized bioreactors and advanced production systems to drive the development of cost-effective, high-quality gene therapies.
-
Vial Breakage During Lyophilization: Root Causes And Mitigation
Learn how controlling freezing rates, using specialized vials, and employing optimized stoppering techniques can reduce the risk of vial breakage during lyophilization and ensure high product quality.
-
Random And Semi-Targeted Integration In Cell Line Development
While semi-targeted approaches may offer precision, uncover how they may fall short in overall efficiency and productivity, especially in late-stage manufacturing.
-
The Latest Trends In The Prevention And Treatment Of Cervical Cancer
What immune checkpoint inhibitors are showing promise in cervical cancer treatments and how are advancements in treatment, paired with additional prevention strategies, improving patient outcomes?
-
Microbial Challenge In-Use Studies
Learn more about how a partner with expertise can help you navigate the complex landscape of microbial challenge in-use studies and ensure the highest standards of patient care.
-
Accelerating ADC Clinical Development Timelines
For ADC developers, obtaining the desired payload can be a challenge, as their synthesis is more complex than most other small molecules. Learn about a more efficient approach to payload synthesis.
PHARMA OUTSOURCING APP NOTES & CASE STUDIES