ISO 22519: An Unnecessary, Faulty, And Confusing Standard

Recently, the PDA Letter published an article titled “ISO 22519: A Flawed and Counterproductive Standard."1 The authors of this article pledged to continue educating industry about deeply flawed ISO Standard 22519,2 which claims to be the new word in purified water (PW) compliance. In fact, this standard neither promotes innovation nor compliance. On the contrary, it brings confusion to those that are embarking on purified water production and use in the pharmaceutical industry. The main proponent of the standard, which effectively acts as a vehicle for the promotion of the proponent’s patented pretreatment technology, is a single manufacturer and its allies. Furthermore, the standard was prepared in the ISO Water Reuse Committee, which has nothing to do with pharmaceutical water.
In response, a team of recognized pharmaceutical water industry leaders has urged the prompt repeal or revision of ISO 22519 based on technical criticism and contradiction with already existing guidance from regulatory bodies. Below is an outline of the team’s criticism, with information quoted from both the ISO 22519 standard and the article3 that promotes the standard. This paper will highlight several critical technical inaccuracies and biases in the standard that render it unusable and irrelevant. Only the most serious flaws in the standard will be discussed here, although there are numerous other technical inaccuracies.
ISO 22519 starts with a claim: “This document provides a standard benchmark that can be used by the industries that use PW and/or WFI [water for injection], national governments, state authorities and regulatory bodies to evaluate PW/WFI systems.” The meaning of this excerpt is clear: it is declaring that regulatory bodies should use this standard as a benchmark by which to evaluate/audit water systems. We should note that there are a great number of guides from the FDA,4 EMA,5 WHO,6 and ISPE,7 not to mention the U.S.,8 European,9 and Japanese10 pharmacopeia that provide such guidance already. Therefore, emergence of this document is counterproductive, as it does not provide a compliant manufacturing instruction and does not promote a risk-based life cycle approach, automatically making it harmful to the industry. Moreover, there are no indications that regulatory bodies were consulted in the preparation of the standard or have reviewed the standard.
The authors of this article also note that the standard is heavy-handed in its use of prescriptive language (“shall” as opposed to “should” or “may”), which renders the standard overly restrictive.
Specific Technical Flaws
Improvement In Water Quality
In the section on Design and Practices, the standard states:
4.2.3 The PW/WFI Pre-treatment and Production water quality shall show improvement in all quality parameters as the water advances through the system.
4.2.4 The following parameters shall be steadily reduced at each stage in the system:
— microbial total count;
— conductivity; and
— TOC.
4.2.5 PW/WFI quality shall be according to the last revision of the local/national/relevant Pharmacopoeia. Table 1 provides recommended water quality.
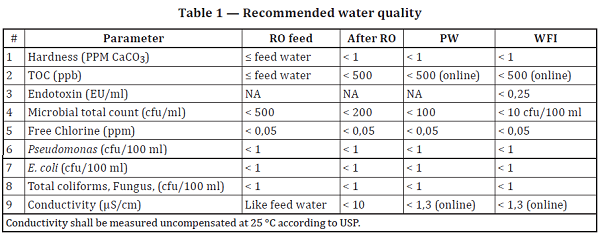
There are number of problems with these paragraphs and tables. The purpose of each purification step, and the order of the steps, is to control various and specific types of impurities, i.e., ions, organics, organisms, particles, etc. Some treatment steps could result in an increase in some attributes while achieving their primary purpose for the others. Adherence to this requirement could also preclude the use of some innovative future technology that does not meet this arbitrary requirement but would be of great value.
- Sections 4.2.3 and 4.2.4 implicitly forbid cation exchange softening, which is one of the most commonly used unit operation for the prevention of hardness scaling of RO membranes:
- There is a slight increase in the conductivity of the water due to the higher equivalent conductance of sodium compared to hardness elements such as calcium and magnesium.
- On very microbially pristine feedwater, it is expected that there may be a slight increase in microbial load due to the large surface area of resin in water ion exchange (IX) softeners.
- IX water softening is a proven technology that is widely used in compendial water treatment and is in use in thousands of water treatment systems worldwide.
- Currently, more than 99 percent of all pharma systems worldwide do not meet these requirements, especially the prescription for “improvement in all quality parameters” in each unit operation. It is necessary that feedwater is first conditioned before the real purification process starts in order to ensure that downstream unit operations will function without being damaged and that water recovery can be optimized. For preconditioning of the water, it is acceptable not to purify the water additionally with each treatment step.
- Sections 4.2.3 and 4.2.4 implicitly forbid the use of activated carbon, which is primarily and extensively used for the neutralization of chlorine and chloramines:
- It is virtually inevitable that when using activated carbon for chlorine (and more importantly chloramine) removal that microbial counts will increase as a result of the removal of disinfection chemicals and the large surface area of activated carbon.
- The standard later indicates that the preferred method (but due to this section, the only viable method) for chlorine removal is UV light treatment. While UV light is a viable technology for chlorine and chloramine removal, it is often not economically or practically the best application choice. Activated carbon has been used successfully in thousands of systems with suitable control strategies to ensure reliability.
- It is virtually inevitable that when using activated carbon for chlorine (and more importantly chloramine) removal that microbial counts will increase as a result of the removal of disinfection chemicals and the large surface area of activated carbon.
- Sections 4.2.3 and 4.2.4 implicitly forbid the use of sodium bisulphite and caustic to neutralize chlorine and to adjust pH, respectively.
- The addition of either of these chemicals will increase conductivity and therefore, they are forbidden.
- As in the case of activated carbon, this treatment option for chlorine removal has been used successfully in hundreds of systems.
- pH adjustment is often utilized in reverse osmosis (RO) pretreatment systems to optimize the CO2-CO32+ and NH3-NH4+ equilibria. Precluding the use of NaOH for pH adjustment would virtually mandate that the removal of these ionizing dissolved gases may only be done with membrane degassing.
- The addition of either of these chemicals will increase conductivity and therefore, they are forbidden.
- Section 4.2.5, which mandates that specific water quality limits be met in the pretreatment, will also likely result in an excessive amount of pretreatment sampling. The sampling is needed to show adherence to the reduction of each parameter cited by each production module. According to industry standards for microbial sampling, each sample costs $250 for sampling, containers, single-use, inoculation, incubation, labor, and analysis. Thus, minimum daily sampling at three pretreatment modules would cost an additional $273,750 per year (i.e., $750 x 365), with no additional compliance with the pharmacopoeia mandates.
USP <1231> states (bold added for emphasis):
“6.4.2 PRETREATMENT AND PURIFICATION SYSTEM SAMPLING
The location and frequency of sampling from ports within the pretreatment and purification systems may be selected based on a risk analysis of unit operation purpose. The purpose of this sampling is primarily for PC (Process Control), for example, to ensure maintenance of acceptable unit operation performance, to assess maintenance procedure efficacy, and to investigate the need for remedial action. Quality deviations in the early portions of the purification process can affect unit operation efficiency but usually do not impact the finished water quality or acceptable use.”
Specifically, USP <1231> states that early portions of the purification process can affect unit operation efficiency, but rarely does it affect the finished water quality or its accepted use. The directive of ISO 22519 to sample each pretreatment module for microbial reduction or ionic reduction will place an onerous burden in cost, labor, laboratory personnel, and laboratory equipment on all biopharmaceutical manufacturers without any compendial mandate compliance.
- Section 4.2.5, Table 1 also misrepresents certain USP requirements:
- The table mandates that conductivity shall be measured at 25°C, while USP allows for uncompensated measurement to be performed at any temperature from 0 to 100֯C. Compensated temperature readings are permissible at 25֯C, according to USP <645> and Eur. Ph. 2.2.38.
- Table 1 mandates that total organic carbon (TOC) be measured online. While it is agreed this is a best practice, it is not a requirement of USP or any other pharmacopeia. See USP <643> and Eur. Ph. 2.2.44, where online or laboratory analysis of the sample is permissible.
- The table implies that E. coli and fecal coliforms are allowed up to 1 cfu/100 ml in PW and WFI. The presence of any E. coli or fecal coliforms is forbidden in the compendial water. USP <1231> states:
“8.2.2 NON-BIOFILM-FORMING BACTERIA IN WATER SYSTEMS
Other types of non-pseudomonad Gram-negative bacteria are generally non-aquatic by nature. They include coliforms from the genera Escherichia, Salmonella, Shigella, Serratia, Proteus, Enterobacter, and Klebsiella, which are used as indicators of fecal contamination. Although some of these bacteria can be human enteric pathogens, these non-pseudomonads are not suited to colonizing or surviving in pharmaceutical water systems owing to the water’s chemical purity. In fact, non-pseudomonad enteric bacteria are extremely unlikely contaminants of pharmaceutical water systems unless local sewage and source water controls are not in place. Such controls are required in order to comply with the source water requirements for making USP-grade waters as described in their respective monographs.”8
Pretreatment Operating Temperature
The standard states:
4.2.7 During production, the PW/WFI Pre-treatment and Production shall control the maximum water temperature in the system. During production, the maximum temperature of the warmest point in the system shall be no more than 25°C (guidance value).
This statement essentially mandates that the pretreatment temperature must not exceed 25° C, but then labels this “shall” level requirement a guidance. At best, this is confusing, and, under a stricter interpretation, it could mean that a pretreatment operating at >25°C is noncompliant, and subject to a regulatory citation. In the USP and Ph. Eur. there is no restriction on the temperature of the water on the pre-treatment. Additionally, in warm climates of India, southeast Asia, southern Asia, and the Middle East, raw water temperature is often greater than 25°C for months at a time. Does this mean biopharmaceutical companies are obligated to provide cooling systems to meet the directive of ISO 22519? Once again, this action has no relevance to the compliance of the pharmacopoeia mandates as written.
Further, this statement prohibits the evolution of continuously hot systems that do not exist today on a commercial basis but are under development.
Membrane Degassing
In the recommended system components/treatment stages the standard suggests regarding the placement of degassing membranes:
5.1.12 Degassing CO2 contact membrane (degasser) – water contact membrane for reduction of CO2 gas in water upstream of RO and CDI/EDI/CEDI.
Typically, it is more effective to install the degassing membrane after the RO in a RO-CEDI system or after the first pass RO in a double pass RO arrangement.
Unit Operation Evaluation
ISO 22519 provides a table in section 5.2 summarizing the advantages and disadvantages of various unit operations. The table is clearly biased towards Electric Scale Control as the best means of mitigating scaling risk in all feed waters. This technology is not endorsed for use by OEM manufacturers of RO membranes, nor CEDI modules as an adequate form of pre-treatment. Although the introduction limits the scope of the standard to exclude “thermal process” it should be made clear in this table that this unit operation is also likely to be completely unusable on vapour compression distillation pre-treatment. In addition, this technology has not been widely tested in the field and it is trademarked, patented and only available from a single proprietary manufacturer.
Pre-treatment Sanitary Design
ISO 22519 essentially requires that the piping in pre-treatment of PW and WFI systems be fully sanitary/hygienic in design while indicating only a 316 L SS piping is adequate.
5.5 Non-final product water piping/tubing in the PW/WFI pre-treatment and production
5.5.1 Only butt welding for piping/tubing welding shall be used.
5.5.2 Butt-welding may be manual or by orbital welding machine. Inspection with borescope and passivation is not required.
5.5.3 Piping/tubing standards shall be 3A/food grade with flange or Tri Clamp (TC) connections.
5.5.4 Piping/tubing internal finish may be polished or standard mill surface finish.
5.5.5 Valves installed may be of the following types: ball, angle, diaphragm, needle and butterfly.
5.5.6 Threaded connections shall not be used.
While a fully hygienic pretreatment design may be a good practice for non-distillation-based WFI systems, this approach is needlessly costly for PW systems; there are hundreds of PW systems in service using non-metallic piping, such as poly vinyl chloride (PVC), polypropylene (PP), CPVC, etc., in the pretreatment that have seen decades of reliable operation.
The spirit of this section is muddled per these three points:
- The section seems to mandate the use of fully hygienic design in pretreatment.
- Then it allows for the use of non-sanitary components:
- The use of flanges in 5.5.3
- The use of ball, needle, and angle valves (which are not of sanitary design) in 5.5.5
- Additionally, if hygienic design is desired, then this section should recommend the pharma industry’s most broadly recognized design standard, the American Society of Mechanical Engineers Bioprocessing Equipment Standard (ASME BPE).11
PW And WFI Piping Design
The standard mandates a high finish:
5.6 Piping/tubing in contact with PW/WFI product
5.6.1 Minimum acceptable ID polish shall be Ra ≤ 0,6 micron.
Literature has shown that there is no appreciable difference in water system microbial performance that has surface roughness between 0.6 µm RA and 0.85 µm RA. Furthermore, this requirement is more restrictive than the World Health Organization recommendation of not greater than 0.8 µm Ra.
Sampling
The standard provides costly and contradictory guidance with respect to sampling point design:
6.1.2 Zero dead leg sample valves shall be used throughout the system, both on non PW/WFI piping/tubing and on product water piping/tubing.
6.1.3 The sample valves shall be installed on short outlet tees so as not to contaminate samples by bioburden growing in the fitting.
Most existing pretreatment systems do not employ zero dead leg (ZDL) valves, and the use of ZDL valves in pretreatment is not required for reliable system operation. In addition, 6.1.3 contradicts 6.1.2, as ZDL valves cannot be installed on short outlet tees.
Required Instrumentation
The standard mandates that flow instrumentation must be used on all RO flow lines:
7.1.4 Flow indicator transmitter (FIT):
— for monitoring the flow rate; and
— flow indicator transmitter (FIT) shall be used at: permeate/product/reject streams.
Especially on smaller RO units, this is not required and there are many systems with flow gauges-only that have operated very reliably for a long time. This should be a user-defined requirement and not a part of a standard to be used to audit systems.
In general, there are other instruments recommended as transmitters that could be done as gauges-only or not at all in some cases and the systems would be perfectly operable; however, the language does not make these other requirements mandatory.
Alarming and monitoring are mandated in Table 2 of ISO 22519 (author emphasis):
7.2 Parameters for measuring, alarming, storing and graphing from online instruments
Table 2 lists the parameters that shall be measured, alarmed, stored and graphed.
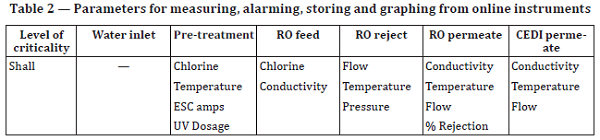
There are two major issues with this table: first, that it is mandatory to measure electrolytic scale control unit amperage, thereby making ESC mandatory. Second, chlorine measurement appears twice, and it may not even be required in European jurisdictions where chlorine is not present.
Additionally, it should not be mandatory from a regulatory perspective to log, trend, and graph several of these parameters, especially RO reject temperature and pressure.
Operation
ISO 22519 prescribes the use of UV for chlorine removal and the use of ESC for scale control:
9.1.5 A system with electric scale control (ESC) and UV dichlorination shall continuously reduce bacteria while operating.
ESC is a patented technology with an extremely limited installed and tested base and is unproven for the ability to control scale at high hardness levels. There are other technologies, including cation exchange softening and the use of antiscalant, that have a long history of successful use.
UV for chlorine and chloramine removal, as stated earlier, may be economically unattractive, resulting in higher than necessary system purchase and operating costs.
Sanitization
Forbidding Chemically Sanitized Systems
The use of non-hot water sanitized systems is precluded in the standard:
4.2.8 All parts of the PW/WFI Pre-treatment and Production shall be hot water sanitized, from the feed water inlet valve to the PW/WFI fill valve. During sanitization, the PW/WFI Pre-treatment and Production shall control the water temperature in the system. During sanitization, all the points of the system should be at a minimum of 80 °C (guidance value).
…
9.3.1 Hot water sanitization shall be the method for keeping the systems clean of microbial contamination.
Again, there are many exclusively chemically sanitized PW systems in operation for decades that have consistently met compendial water requirements. This requirement measurably increases the cost (capital and operating), energy consumption, and complexity of PW pretreatment without an appreciable decrease in risk to product or patient safety at a time when manufacturers (especially generics firms) are being asked to reduce manufacturing costs and reduce energy consumption.
Activated Carbon Sanitization
The guide mandates that steam sanitization be used for activated carbon:
9.3.6 Activated Carbon Filter (ACF) steam sanitization shall be performed with Pure steam (PS) only; industrial steam for direct Activated Carbon Filter (ACF) contact sanitization shall not be used.
It is known that steam sanitization of carbon beds is ineffective due to channelling in the carbon bed and that the best way to sanitize carbon beds is to use hot water.12
Background On ISO 22519 Preparation
The preparation of ISO 22519 had limited participants. Input for this standard was extremely limited and there was no public review process followed in preparation of this standard. Interestingly, the ISO committee responsible for preparation of this standard is TC282, Water Reuse. Most of the members of this committee have little or no experience in pharmaceutical water treatment.
Conclusions
The ISO 22519 standard does not reflect current accepted or even future best practices and is deeply flawed and biased against proven water treatment unit operations in favor of a trademarked technology.
Adherence to this standard will not result in improved patient safety, but will result in design limitations, reduction in competitive bidding, and an increase in the total cost of water production for pharmaceutical purposes. This will be especially true for dosage forms that require large quantities of water in the manufacturing process (e.g., biotech, liquid, and semisolid forms). As manufacturing costs per unit increase, the public will face increased costs for necessary pharmaceuticals.
If plants are inspected by authorities to strictly comply with ISO 22519, the vast majority will fail and, if closed down, there will be a massive drug shortage on all drugs produced globally.
The authors and signatories to this paper recommend that this standard be revoked and not be recognized by regulatory bodies, owners of pharmaceutical and biotechnology manufacturing facilities, manufacturers of water treatment equipment, and engineering firms.
As stated earlier in this article, there are numerous guidance documents from U.S., European, and Japanese pharmacopoeia; ISPE; and a variety of other organizations that provide recognized guidance on how to successfully design, build, and operate compendial water treatment systems. In addition, these listed guidances serve as references for inspection of water systems around the world. Therefore, there is no need for a new standard that does not follow recognized best practices and furthermore contains numerous engineering, microbiological, and compliance errors, inaccuracies, and mistakes. This team of authors will continue to advocate against the adoption of this dubious standard.
References:
- “ISO 22519: A Flawed and Counterproductive Standard,” PDA Letter, August/September 2019 (https://www.pda.org/pda-letter-portal/home/full-article/iso-22519-a-flawed-and-counterproductive-standard)
- ISO, ISO 22519: Purified water and water for injection pretreatment and production systems, June 2019 (https://www.iso.org/standard/73381.html)
- Sackstein, S. “New ISO Standard Available for Water Systems,” PDA Letter, June/July 2019 (https://www.pda.org/pda-letter-portal/archives/full-article/new-iso-standard-available-for-water-systems).
- FDA, Guide to Inspections of High Purity Water Systems, July 1993 (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/high-purity-water-system-793)
- EMA, Guideline on the quality of water for pharmaceutical use (draft), November 2018 (https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-water-pharmaceutical-use_en.pdf)
- World Health Organization, Annex 2: WHO good manufacturing practices for pharmaceutical products: main principles, 2014 (https://www.who.int/medicines/areas/quality_safety/quality_assurance/TRS986annex2.pdf)
- ISPE, Baseline Guide, Volume 4: Water and Steam Systems, 3rd Edition, September 2019 (https://ispe.org/publications/guidance-documents/baseline-guide-vol-4-water-steam-systems-3rd-edition)
- United Sates Pharmacopeia National Formulary, (www.usp.org/USPNF)
- European Directorate for the Quality of Medicines & HealthCare, European Pharmacopoeia, 9th Edition (https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition)
- Pharmaceuticals and Medical Devices Agency, Japanese Pharmacopoeia, 17th Edition (https://www.pmda.go.jp/english/rs-sb-std/standards-development/jp/0019.html)
- American Society of Mechanical Engineers, Bioprocessing Equipment Standard – BPE 2019 (https://www.asme.org/codes-standards/find-codes-standards/bpe-bioprocessing-equipment-(1))
- Collentro, W.V. “USP Purified Water Systems: Discussion of Pretreatment – Part II,” Pharmaceutical Technology, May 1994
Authors and Signatories:
| Name | Author / Signatory | Organization |
| Anders Widov | Author | WIPHE |
| Anthony Bevilacqua | Author | Mettler Toledo |
| Brian Hagopian | Author | Clear Water Consulting |
| Brian Pochini | Author | Sanofi |
| Fritz Roeder | Author | Merck |
| Gary Zoccolante | Author | Plymouth Rock Water Consultants |
| Igor Gorsky | Author | Concordia Valsource / PDA |
| Joe Manfredi | Author | GMP Systems |
| Nik Krpan | Author | Cheme Engineering Inc |
| Nissan Cohen | Author | Biopharmaceutical Water Doc |
| Paolo Leani | Author | Stilmas |
| Sanjay Kolawale | Author | Austar |
| Stephan Neumann | Author | Boehringer Ingleheim |
| TC Soli | Author | Soli Pharma Solutions |
| William V. Collentro | Author | Pharmaceutical Water Specialists, LLC |
| Chris Peterson | Signatory | Abbvie |
| Glyn Davies | Signatory | NNE |
| Jeff Okun | Signatory | Aqua Chem |
| Juha Mattila | Signatory | Steris |
| Kim Klein | Signatory | Meco |
| Mika Parkka | Signatory | Steris |
| Robert P. Vecchione | Signatory | BWT Pharma & Biotech Inc |
