How GSK Launched A Continuous Manufacturing Pilot Plant — And What It Learned
Last November, Outsourced Pharma had the privilege of visiting GSK’s U.S. global R&D hub in Upper Providence, Pa. The 1.4-million sq. ft. facility will soon house the company’s consolidated U.S. R&D activities across the areas of HIV and infectious disease, oncology, immune-inflammation, metabolic pathways and cardiovascular, and dermatology. By 2018, it will be home to more than 3,200 employees, who will collaborate on target identification, safety studies, active pharmaceutical ingredient (API) manufacture, and many other R&D-related activities.
 One of the highlights of the day was a tour of the facility’s continuous manufacturing (CM) pilot plant, led by senior process engineer Scott Cheeseman from GSK’s Platform & Technology Science group. Cheeseman spent 23 years at Merck in API pilot plants and API development before joining GSK in 2014, where he was assigned to lead the design, installation, commissioning, and qualification of the Upper Providence continuous pilot plant.
One of the highlights of the day was a tour of the facility’s continuous manufacturing (CM) pilot plant, led by senior process engineer Scott Cheeseman from GSK’s Platform & Technology Science group. Cheeseman spent 23 years at Merck in API pilot plants and API development before joining GSK in 2014, where he was assigned to lead the design, installation, commissioning, and qualification of the Upper Providence continuous pilot plant.
Outsourced Pharma recently followed up with Cheeseman for more in-depth information about GSK’s continuous manufacturing initiatives, the hurdles it faced in bringing the pilot plant online, the lessons it learned from the experience, and what it plans to do next with the technology.
Please provide some background information on GSK’s continuous manufacturing initiatives.
The way I understand it, flow chemistry and continuous processing within GSK has been an up-and-down adventure over the last 10 to 15 years. The company spent a lot of time developing its first flow plant, which was installed about 10 years ago in Stevenage, UK (now the site of GSK’s other global R&D hub). Our first commercial process came out of that pilot plant — we have built a lot of expertise there.
Since I joined the company, GSK has made a commitment to move toward continuous processing. The idea is to apply as much continuous processing as possible to new drug filings.
Also during the last three years, GSK installed a continuous process in our Global Manufacturing & Supply (GMS) facility in Singapore, which is now up and running.
And I was responsible for the installation of a pilot plant for small molecule, organic, continuous flow chemistry here in Upper Providence for R&D. We recently worked hand-in-hand with operations, engineers, R&D chemists, and other staff members to run the plant for 24 hours per day upwards of 60 percent of the time for eight straight weeks. The project will help support the filing of what will likely be the first drug substance developed in flow by first intent.
Tell us more about the Upper Providence flow plant.
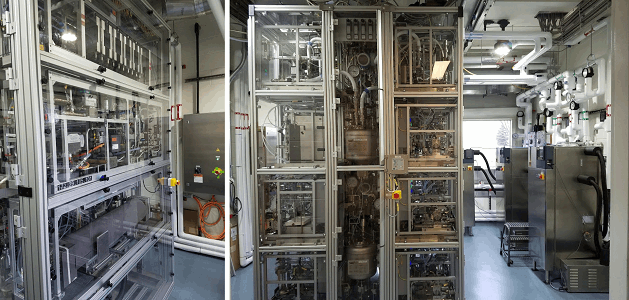
This is GSK’s playground to demonstrate processes designed on the benchtop by first intent, prior to technically transferring them to another facility.
It was designed to be as flexible as possible — to be able to host any future unknown piece of continuous processing equipment for small molecule organic flow chemistry. So as technology evolves, the plant will be able to host it without any major modification.
When you walk through a traditional batch API facility, the equipment is big. It’s permanent. It’s not coming out of the facility without literally taking walls down and tearing it out of the floors.
This pilot plant was designed to come apart. It was designed without any specific equipment in it, but to allow us to install equipment as needed. For example, the first process we just ran on the plant required many micro annular gear pumps; the plant was designed so we can take those in and out, change them over quickly. We ended up having to do a separation, and we were able to quickly install a thin film evaporator. We can put in equipment to control back pressure. We can add multiple types of flow reactors. During the last eight weeks of processing, we had to reconfigure the plant probably four times, at a minimum — and each configuration was completed in less than eight hours.

Need to update your facilities design? Learn the basic principles of facility layout that allow your operations to scale without pain by attending Herman Bozenhardt's webinar:
Bioprocess Facility Design- Layout Rules and Configurations
Currently, the plant is commissioned and ready for all process development activities. We purposefully ran our first process as a development campaign so we could learn the best practices required to put together meaningful standard operating procedures (SOPs), emergency operating procedures (EOPs), instructions, etc.
Speaking of which, what were some of lessons you learned during that initial eight-week run?
Probably one of the biggest learnings was that you have to be very careful with respect to safety and industrial hygiene. You need to make sure you understand exactly how to take the equipment apart for each specific process. That’s something you can only learn by doing, and I would say we’ve learned how to do it better on several occasions.
Another significant learning was what it would take to run the facility — the manpower requirements and the hour requirements, both of which were higher than what people were ready for. But in the long run, it will be a lot easier to operate this plant. . For instance, the amount of material you can make in a given amount of time increases dramatically versus a batch facility.
One final learning was how quickly we could gather data for so many different conditions. In a matter of weeks, we were able to conduct tests on about four dozen reaction conditions. We had two reactions telescoped, followed by an evaporation step — all continuously. In literally two weeks of processing out of eight, we were able to gather information about 40 different sets of operation conditions, which is unprecedented when it comes to gaining that type of experience on a plant.
Aside from being able to gather process data so quickly, what other benefits has GSK realized from
In my opinion, the biggest benefit is being able to demonstrate internally that the technology is here and it’s now, because one of the biggest challenges is changing mindsets from batch development to first intent continuous development. So one of our main goals was to get a first success out there and show people that it can be done.
Another benefit is that processing continuously produces a huge amount of data. We use an infrared flow cell for one of the reactions, online conductivity for another, online high-performance liquid chromatography (HPLC) for another unit operation, and so on. This PAT gathers a lot of real-time data you can use to quickly identify trends through certain condition changes. You know almost instantly what's going on in the plant, whether the material being produced is acceptable, whether flow rates are going the way they should be, etc. Anything that would be indicative of the manufacturability or quality of the product and process, we literally have at our fingertips.
This plant has provided the flexibility to rapidly test a wide range of process conditions, allowing us to develop a better control strategy for transfer to a supplier organization. We were able to quickly stretch all of the potential critical processing parameters to see how the overall process behaved. So we have a really good idea of where the center line conditions are, and what we can tolerate when it comes to making good material.
What are the next steps for CM at GSK?
Our plant is at the forefront of where GSK is going with continuous small molecule flow chemistry. Anything that goes from a lab to a plant will come through our shop, so we can basically try to break the process — learn about control strategies, learn about operability, and de-risk and improve the process here before we send it to a supply organization, most likely within GSK. As development of continuous organic flow chemistry goes at GSK, so will the use of this plant here.
The next step will be qualifying this plant so it can do GMP runs as well. However, the goal for now is to take this experience we just gained and use it as a first success to help change the mindsets in R&D. We want to build confidence so that process development can rely on the technologies of continuous processing, instead of staying with the comfortable intent of developing in batch processing.
I definitely think we demonstrated that flexible and agile manufacturing isn’t just a dream. It’s something that we can use to get information more quickly, help speed the entire development process, and better inform any tech transfer that we ultimately have to do. And if the design is repeated in actual manufacturing facilities, you could manufacture almost anywhere in the world with this type of plant, and you wouldn’t need that much infrastructure to do it.
From an industry perspective, where do you think CM will go from here?
I think everyone in the industry is collectively waiting to see how the regulatory agencies respond to the technology and what guidance it develops. The agencies are very open to continuous manufacturing, but there are a lot of questions they haven’t answered. For example, with control strategies, what does it mean to be in control? How much do you have to manufacture to demonstrate you are producing good material? Can I operate the equipment in a way that is predictive of success?
However, I also think that more companies will have to take chances and implement CM while working with regulatory agencies. We won’t get anywhere unless we continue to put examples out there for the regulators to review.
