Analysis Of The Non-alcoholic Steatohepatitis (NASH) Drug Pipeline & Market: Sizing Up The First Wave
By Mavra Nasir, Ph.D., Crystal Hsu, and Peter Bak, Ph.D., Back Bay Life Science Advisors

Non-alcoholic steatohepatitis (NASH)1 is the advanced form of non‑alcoholic fatty liver disease (NAFLD), a chronic disease marked by excessive fat accumulation in the liver. The growing global burden of NASH and NAFLD is indisputable, with the prevalence of NAFLD expected to grow to ~30% by 2030.2
For pharmaceutical companies, NASH has remained a relative graveyard of drug development over the past 40 years: no disease-specific approved therapies have made it to market. The NASH pipeline is littered with failures in late-stage trials — including the FDA’s recent rejection of the much-anticipated farnesoid X receptor (FXR) agonist, obeticholic acid (OCA, Intercept Pharmaceuticals) — which is likely to impact other companies developing NASH therapeutics. While some companies with programs that have demonstrated a positive risk-benefit profile may be unfazed by this, others will likely require a strategic repositioning to maximize value for their assets in the near term.
Back Bay Life Science Advisors analyzed the first wave of drugs (at Phase 3) poised to change the NASH management paradigm and shape the ambiguous pricing and reimbursement landscape. We also looked at the most promising second wave of drugs (at Phase 2), many of which already demonstrated improved safety and/or efficacy in topline data released to date. In addition, we examined the historical transaction landscape for NASH, identifying key trends for companies looking to build their pipeline and address the multifaceted nature of the disease. Last, we analyzed the potential market access dynamics that will complicate pricing for a therapy in this space given the lack of marketed analogs and uncertainty over the clinical relevance of surrogate endpoints.
This article is the first in a three-part series based on our research. Together, these articles provide an overview of the most promising NASH pipeline candidates, associated efficacy and safety data, the NASH transaction landscape, and market access dynamics.
The Silent Epidemic
Nonalcoholic fatty liver disease (NAFLD) is a continuum of liver pathologies ranging from simple hepatic steatosis (>5% fat liver content, also known as nonalcoholic fatty liver (NAFL)) to NASH. In contrast to NAFL, NASH is associated with varying degrees of hepatocyte inflammation, ballooning, and fibrosis (F1-F3).
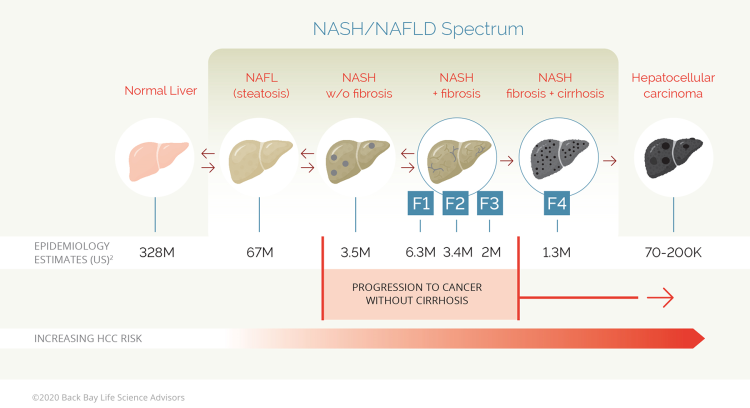
Figure 1: The progression of a healthy liver to NAFLD and NASH-related pathologies
Approximately 20 to 25% of patients with NASH F1-F3 fibrosis can progress to cirrhosis (F4) within a decade, although the rate may vary depending on underlying comorbidities.3,4 Subsequent hepatocellular carcinoma (HCC) development is reported in ~12.8% of patients with cirrhosis and fibrosis (<3 years), although HCC may also manifest in the absence of liver fibrosis and cirrhosis.5 Consequently, the extent of fibrosis is universally considered a strong predictive factor for correlating the progression of NAFLD with life-threating complications.
Several factors contribute to the progression to HCC; these include modifiers such as genetic (PNPLA3, TM6SF2)6,7 and environment (high caloric diet, sedentary lifestyle)8–11 and comorbidities such as obesity (BMI > 30kg/m2), insulin resistance, and/or Type 2 diabetes.12–15 The underlying metabolic derangements are multifactorial and include insulin resistance,16–21 immune and inflammation dysregulation,22–26 impaired free oxygen radical scavenging,27,28 deficiencies in mitochondrial structure and function,17,29 enhanced hepatic iron,30,31 and hepatotoxic byproducts of gut bacteria.32,33
In the U.S., the reported prevalence of NAFLD is ~10 to 46%, with most biopsy-based studies reporting a NASH prevalence of ~3 to 5%, resulting in an adult prevalent NASH population of ~3.9 million to 19.7 million.34–36 The expected prevalence of NAFLD and NASH is expected to increase in the U.S. and internationally for at least another decade,2,37 with one panel of international experts dubbing NAFLD as the “silent epidemic.”38 Their concerns are substantiated by recent data, highlighting the burden of NAFLD in young adults (n=4,201, 18 to 24 years) with obesity identified as the driving risk factor.
While the majority of NASH patients are diagnosed in their 40s to 50s, Abeysekera et al. used transient elastography to show that 20% of young adults had steatosis and ~2.7% had fibrosis (F2–F4) by 24 years of age.39 Given that rates of obesity are gradually increasing over time,40 morbidity and mortality due to progressive NAFLD will likely manifest at a younger age, leading to significant clinical and economic burden over the next decades.
Diagnosis And Current Treatments
As most patients with NAFLD remain asymptomatic, laboratory tests showing high liver aminotransferases or hepatic steatosis incidental to abdominal imaging is the more common path to diagnosis. Liver biopsy is the gold standard method for diagnosis of NASH, with biopsy confirmed endpoints required for FDA and European Medicines Agency (EMA) approval of NASH candidates.
The primary treatment for NASH now is lifestyle modification through diet and exercise, with the ultimate goal of reducing weight.41,42 However, the degree of weight loss required for histologic improvement of NASH may be difficult to achieve and even harder to sustain for patients. In some cases, bariatric surgery remains is the only effective weight-loss therapy43–45 and has also demonstrated gains in cardiovascular outcomes,46 the leading cause of premature mortality in patients with NAFLD.47,48
For patients with biopsy-proven NASH and fibrosis score but without diabetes, vitamin E (800 international units per day) may be used, although evidence is limited. In patients with NASH and diabetes, metformin and/or pioglitazone/liraglutide may be prescribed. However, given the lack of FDA approved NASH pharmacological interventions, there is a high clinical need for effective drugs.
Nash Drugs: The First Wave
At peak, the global NASH market is expected to hit an average of $13 billion annually by 2030.49 As of June 2020, the NASH pipeline of first-wave drugs holds 54 clinical candidates in development by 47 companies that are evaluating 29 different mechanisms of action. Over the last two decades, the NASH pipeline has seen many late-stage programs fail to show clinical efficacy, despite targeting a broad range of mechanisms of action.
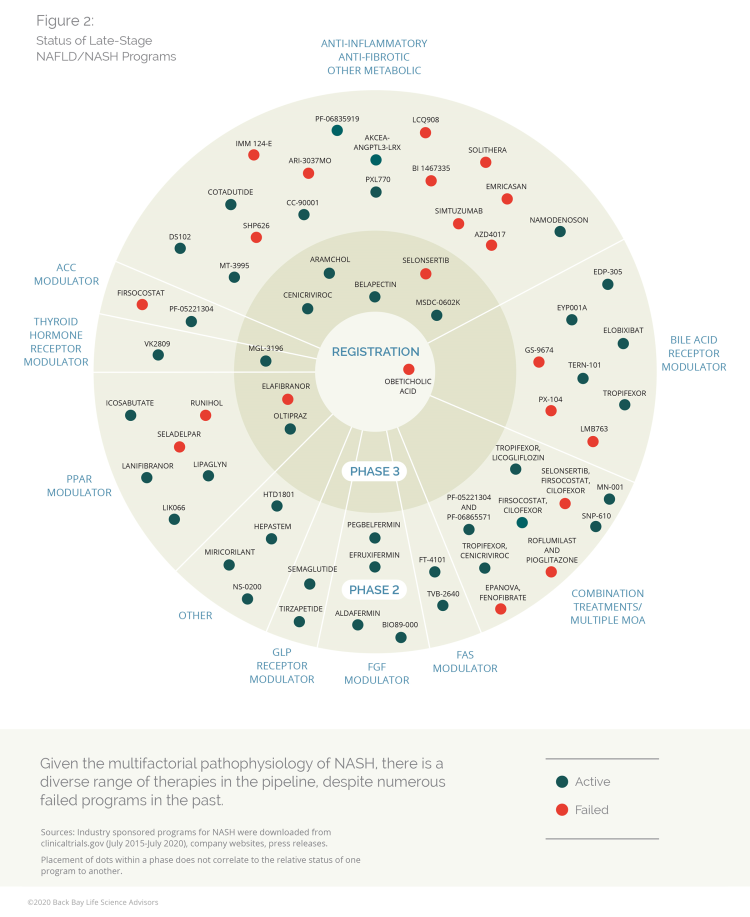
Figure 2: Status of late-stage NAFLD/NASH programs (Click here for more comprehensive information about late-stage NAFLD/NASH programs, including phase, status, MOA, drug, and company.)
Competition to be the first marketed NASH drug remains high, with several late-stage candidates vying for the opportunity. However, lack of harmony between the FDA and the EMA complicates the development pathway for NASH:50 While the FDA only requires the achievement of one NASH endpoint (improvement of ≥1 stage in fibrosis with no worsening of NASH or improvement in NASH resolution with no worsening of fibrosis)51, the EMA’s draft guidance requires efficacy in both these endpoints.52 This will likely limit or delay approvals of first movers in the major European markets.
The first wave of NASH candidates includes:
Obeticholic acid (Intercept Pharmaceuticals)
Intercept’s application for accelerated approval for obeticholic acid (OCA) was denied by the FDA on June 29, 2020, contrary to expectations.53 OCA is approved for primary biliary cholangitis (PBC) under the name Ocaliva, providing Intercept with an opportunity to familiarize physicians with the drug prior to launch in NASH. In the pivotal study (REGENERATE) OCA demonstrated improvement in liver fibrosis (>1 stage) without worsening of NASH at 18 months in the treatment vs. placebo group (23% vs. 12%)54

Figure 3: Comparison of efficacy data (fibrosis improvement NASH resolution)
However, concerns remained about OCA’s side effects: 51% of patients experienced pruritis (with 9% terminating treatment) and 20% of patients required statins due to elevations in low-density lipoprotein cholesterol (LDL-C). After reviewing the data submitted for accelerated approval, the FDA deemed the demonstrated efficacy to be “modest” and not strong enough to warrant safety risks. Intercept has a year to respond to the agency’s concerns and is scheduled to present additional data at liver conferences over the next months. However, the company recently cut the workforce by an estimated 25%.55
Although Intercept’s first mover advantage with OCA as a monotherapy for NASH is now at risk, the drug may still be part of the first wave of therapies to hit the market. The company presented additional data from the REGENERATE study post-FDA rejection at the International Liver Congress 2020. Sustained improvement in non-invasive biomarkers (alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), serum markers of fibrosis (FIB-4, AST to platelet ratio index [APRI]), and liver stiffness (FibroScan vibration-controlled transient elastography [VCTE]) were associated with changes in histologic fibrosis.56
Moreover, combinatorial studies may be on the horizon, although likely delayed given the FDA setback. In 2019, Intercept entered into an undisclosed licensing deal with Aralez Pharmaceuticals for the pan-PPAR agonist bezafibrate.57 The company is currently planning to investigate the efficacy of OCA and bezafibrate in patients with PBC, and the future expansion to NASH would not be out of the question given the complexity of the disease. Even with Elafibranor’s (PPAR-alpha and PPAR-delta agonist Genfit) recent failure in Phase 3,58 Intercept may consider pursuing development of bezafibrate in NASH post-PBC given the promising results from the pan-PPAR agonist Lanifibranor (Inventiva) released in June 2020.59
Last, data from the REVERSE trial evaluating OCA in compensated NASH patients (F4) have yet to be released, with trial enrollment completed in January 2020. Although NASH patients with cirrhosis represent a smaller subset of the NASH addressable market (~1 million),2 morbidity and mortality are higher, with approximately twice the annual inpatient ($61,000 vs. $34,000) and out-patient charges ($12,000 vs. $8,800) compared to patients without cirrhosis.60
MGL-3196, Resmetirom (Madrigal Pharmaceuticals)
Currently in Phase 3 trials with target enrollment exceeded as of September 2020, Resmetirom has demonstrated the strongest clinical efficacy to date (29% vs. 12% ≥1 stage fibrosis improvement, treatment versus placebo) in Phase 2 studies of NASH (see Figure 3).61
The (THR) β-selective agonist’s strong ability to reduce hepatic fat and multiple atherogenic lipids (LDL cholesterol, apolipoprotein B, triglycerides, lipoprotein) suggests a possible cardiovascular benefit, which is highly desirable in the NASH population.62 Data presented at the summit of The European Association for the Study of the Liver (EASL) 2020 showed that once-daily oral 80 mg and 100 mg doses of Resmetirom led to ≥50% and ≥60% reductions in liver fat, respectively, and were associated with a statistically significant 64% NASH resolution.63
Resmetirom led to a statistically significant reduction in markers of net collagen deposition in the liver, supporting the anti-fibrotic action of Resmetirom. Despite the strong correlation observed between patients experiencing high-fat reductions measured by magnetic resonance imaging proton density fat fraction (MRI-PDFF) and histological biopsy data, the FDA would require biopsy data to make approval decisions. Familiarizing liver specialists with MRI-PDFF and pursuing incorporation of the procedure into U.S. and European clinical guidelines will likely drive broad clinical adoption.
While results of Phase 2 (n=125) have yet to be repeated in the much larger Phase 3 study (n=2,000), the company is well positioned in the NASH space given the strong risk-benefit profile. Compared to most other manufacturers in the space, Madrigal has not announced any trials for F4 patients, which may restrict use to F2–F3 patients. Pursuing a strategic partnership with a large pharma company for further clinical development within this population should be prioritized to de-risk value for the program.
Cenicriviroc (Allergan)
After acquiring Tobira Therapeutics in 2016 for ~$1.7 billion, Allergan entered the NASH race by adding Cenicriviroc (CVC) to its developmental pipeline.64 Even though CVC has a differentiated mechanism of action compared to OCA and Resmetirom, weaker efficacy data compared to both those candidates have reduced confidence in the drug’s efficacy as a monotherapy (see Figure 3).65 Not surprisingly, Allergan has teamed up with Novartis to test CVC in tandem with the latter’s FXR agonist Tropifexor in a Phase 2b study that could combine CVC’s anti-inflammatory effects with Tropifexor’s anti-lipogenesis profile.66 Furthermore, Allergan acquired multiple FXR agonists after its 2016 buyout of Akarna Therapeutics for $50 million up front,67 and the potential to run late-stage clinical trials in the F4 population provides a potential favorable developmental path forward for CVC.
Aramchol (Galmed Pharmaceuticals)
Aramchol is a novel, first in class SCD1 modulator, aiming to reduce liver fat and collagen production. While the Phase 3 ARMOR study is currently recruiting patients, the Phase 2b ARREST study data demonstrated significant reductions in liver enzymes (aspartate transaminase and aspartate aminotransferase) and hemoglobin A1c compared to placebo.68 Although the biochemical improvement and glycemic control is a desirable trait, the drug failed to demonstrate statistically significant improvement in either of the FDA required endpoints [Table 2].68
The primary endpoint of reduction in mean liver fat in the Phase 2b study was higher in the lower dose group (400 mg vs placebo) compared to the higher dose (600 mg vs. placebo), with the higher dose not reaching statistical significance. Like Resmetirom’s Phase 2 study (n = 247), the sample sizes are relatively small, and conclusive evidence will be dependent on results from the larger Phase 3 studies (n = 2,000).
To further strengthen the program, Galmed announced a licensing and share purchase agreement with One Way Liver Genomics for the development of a non-invasive, blood-based companion diagnostic tool for Aramchol.69
Belapectin (Galectin Therapeutics)
Galectin’s lead NASH agent is uniquely positioned in pursuing a label for the management of cirrhotic NASH patients without esophageal varices. While the Phase 2b study failed to show a reduction in portal hypertension (hepatic venous pressure gradient [HVPG] ≥ 6 mm Hg) or improvement in fibrosis, a subgroup analysis of patients without esophageal varices did show statistically significant decrease in HVPG and development of varices.42
Galectin decided to pursue further development in this subgroup and after receiving the FDA’s nod on an adaptive trial design, started enrollment in June 2020. Given that NASH patients with cirrhosis (F4) represent a subset (~1 million) of the larger NASH F1-F3 population,2 and there are no signs of efficacy in the former, Galectin will need to significantly de-risk the program to attract partners.
In Part 2 of our three-part series on NASH development, we will highlight the promising second wave of NASH drugs, many of which have demonstrated improved safety and/or efficacy compared to the first wave.
References:
- Ludwig, J., Viggiano, T. R., Mcgill, D. B. & Oh, B. J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. in Mayo Clinic Proceedings vol. 55 434–438 (1980).
- Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology (2018) doi:10.1002/hep.29466.
- Ekstedt, M. et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology (2006) doi:10.1002/hep.21327.
- Matteoni, C. A. et al. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology (1999) doi:10.1016/S0016-5085(99)70506-8.
- White, D. L., Kanwal, F. & El-Serag, H. B. Association Between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clin. Gastroenterol. Hepatol. (2012) doi:10.1016/j.cgh.2012.10.001.
- Romeo, S. et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. (2008) doi:10.1038/ng.257.
- Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. (2014) doi:10.1038/ng.2901.
- Gerber, L. et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: A population-based study. Aliment. Pharmacol. Ther. (2012) doi:10.1111/apt.12038.
- Lanaspa, M. A. et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. U. S. A. (2018) doi:10.1073/pnas.1713837115.
- Jensen, T. et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. Journal of Hepatology (2018) doi:10.1016/j.jhep.2018.01.019.
- Leslie, T. et al. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann. Hepatol. (2014) doi:10.1016/s1665-2681(19)31253-0.
- Powell, E. E. et al. The natural history of nonalcoholic steatohepatitis: A follow‐up study of forty‐two patients for up to 21 years. Hepatology (1990) doi:10.1002/hep.1840110114.
- Younossi, Z. et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology and Hepatology (2018) doi:10.1038/nrgastro.2017.109.
- Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (2016) doi:10.1002/hep.28431.
- Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nature Reviews Gastroenterology and Hepatology (2013) doi:10.1038/nrgastro.2013.41.
- Utzschneider, K. M. & Kahn, S. E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. Journal of Clinical Endocrinology and Metabolism (2006) doi:10.1210/jc.2006-0587.
- Sanyal, A. J. et al. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology (2001) doi:10.1053/gast.2001.23256.
- George, J. et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology (2002) doi:10.1053/jhep.2002.30692.
- Cavallo-Perin, P. et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology (2002) doi:10.1053/jhep.2002.30690.
- Hamaguchi, M. et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. (2005) doi:10.7326/0003-4819-143-10-200511150-00009.
- Marchesini, G. et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. (1999) doi:10.1016/S0002-9343(99)00271-5.
- Wree, A. et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. (2014) doi:10.1007/s00109-014-1170-1.
- van der Windt, D. J. et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology (2018) doi:10.1002/hep.29914.
- Baeck, C. et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut (2012) doi:10.1136/gutjnl-2011-300304.
- Yu, Y. et al. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J. Clin. Invest. (2019) doi:10.1172/JCI121842.
- Vespasiani-Gentilucci, U. et al. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver Int. (2015) doi:10.1111/liv.12531.
- Baskol, G., Baskol, M. & Kocer, D. Oxidative stress and antioxidant defenses in serum of patients with non-alcoholic steatohepatitis. Clin. Biochem. (2007) doi:10.1016/j.clinbiochem.2007.02.006.
- Ikura, Y. et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: Impact on disease progression. Hepatology (2006) doi:10.1002/hep.21070.
- Nocito, A. et al. Serotonin Mediates Oxidative Stress and Mitochondrial Toxicity in a Murine Model of Nonalcoholic Steatohepatitis. Gastroenterology (2007) doi:10.1053/j.gastro.2007.05.019.
- Facchini, F. S., Hua, N. W. & Stoohs, R. A. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology (2002) doi:10.1053/gast.2002.32403.
- Adams, L. A. et al. The impact of phlebotomy in nonalcoholic fatty liver disease: A prospective, randomized, controlled trial. Hepatology (2015) doi:10.1002/hep.27662.
- Puri, P. & Sanyal, A. J. The Intestinal Microbiome in Nonalcoholic Fatty Liver Disease. Clinics in Liver Disease (2018) doi:10.1016/j.cld.2017.08.009.
- Loomba, R. et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. (2017) doi:10.1016/j.cmet.2017.04.001.
- Vernon, G., Baranova, A. & Younossi, Z. M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Alimentary Pharmacology and Therapeutics (2011) doi:10.1111/j.1365-2036.2011.04724.x.
- Williams, C. D. et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology (2011) doi:10.1053/j.gastro.2010.09.038.
- Lazo, M. et al. Prevalence of nonalcoholic fatty liver disease in the United States: The third national health and nutrition examination survey, 1988-1994. American Journal of Epidemiology (2013) doi:10.1093/aje/kws448.
- Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. (2018) doi:10.1016/j.jhep.2018.05.036.
- Lazarus, J. V. et al. NAFLD — sounding the alarm on a silent epidemic. Nature Reviews Gastroenterology and Hepatology (2020) doi:10.1038/s41575-020-0315-7.
- Abeysekera, K. W. M. et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol. Hepatol. (2020) doi:10.1016/S2468-1253(19)30419-4.
- Swinburn, B. A. et al. The global obesity pandemic: Shaped by global drivers and local environments. The Lancet (2011) doi:10.1016/S0140-6736(11)60813-1.
- Marchesini, G. et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. (2016) doi:10.1016/j.jhep.2015.11.004.
- Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (2018) doi:10.1002/hep.29367.
- Bray, G. A. The missing link - Lose weight, live longer. New England Journal of Medicine (2007) doi:10.1056/NEJMe078135.
- Sjöström, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. (2007) doi:10.1056/NEJMoa066254.
- Adams, T. D. et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. (2007) doi:10.1056/NEJMoa066603.
- Moussa, O. et al. Effect of bariatric surgery on long-term cardiovascular outcomes: a nationwide nested cohort study. Eur. Heart J. (2020) doi:10.1093/eurheartj/ehaa069.
- Torres, D. M. & Harrison, S. A. Nonalcoholic fatty liver disease: Fibrosis portends a worse prognosis. Hepatology (2015) doi:10.1002/hep.27680.
- Adams, L. A. et al. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology (2005) doi:10.1053/j.gastro.2005.04.014.
- Bell, J. No one knows the size of the NASH market. BioPharma Dive https://www.biopharmadive.com/news/no-one-knows-the-size-of-the-nash-market/554240/ (2019).
- Brennan, Z. Drugmakers Seek US, EU Harmonization on NASH Drug Development Guidance. Regulatory Affairs Professionals Society https://www.raps.org/news-and-articles/news-articles/2019/8/drugmakers-seek-us-eu-harmonization-on-nash-drug (2019).
- US Food and Drug Administration. Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/noncirrhotic-nonalcoholic-steatohepatitis-liver-fibrosis-developing-drugs-treatment (2018).
- European Medicines Agency. Draft reflection paper on regulatory requirements for the development of medicinal products for chronic non-infectious liver diseases (PBC, PSC, NASH). https://www.ema.europa.eu/en/draft-reflection-paper-regulatory-requirements-development-medicinal-products-chronic-non-infectious (2018).
- Intercept Pharma. Intercept receives complete response letter from FDA for obeticholic acid for the treatment of fibrosis due to Nash. Press Release https://ir.interceptpharma.com/news-releases/news-release-details/intercept-receives-complete-response-letter-fda-obeticholic-acid (2020).
- Intercept Pharma. Intercept announces positive topline results from pivotal Phase 3 regenerate study of obeticholic acid in patients with liver fibrosis due to Nash. Press Release https://ir.interceptpharma.com/news-releases/news-release-details/intercept-announces-positive-topline-results-pivotal-phase-3 (2019).
- Intercept Pharma. Intercept Securities Exchange Form 8-K. Press Release https://ir.interceptpharma.com/static-files/888c1844-8eb9-4bf7-9ba6-6486e377cde9 (2020).
- Intercept (ICPT) Announces New Data from Interim Analysis of REGENERATE Show OCA Helped Patients with Liver Fibrosis Due to NASH Achieve Sustained Improvement. StreetInsider https://www.streetinsider.com/Corporate+News/Intercept+%28ICPT%29+Announces+
New+Data+from+Interim+Analysis+of+REGENERATE+Show+OCA+Helped+Patients
+with+Liver+Fibrosis+Due+to+NASH+Achieve+Sustained+Improvement/17293586.html (2020). - Intercept Announces NASH and PBC Program Updates. GlobeNewswire https://www.globenewswire.com/news-release/2019/01/07/1681125/0/en/Intercept-Announces-NASH-and-PBC-Program-Updates.html (2019).
- Genfit. GENFIT: Announces Results from Interim Analysis of RESOLVE-IT Phase 3 Trial of Elafibranor in Adults with NASH and Fibrosis. Press Release https://ir.genfit.com/news-releases/news-release-details/genfit-announces-results-interim-analysis-resolve-it-phase-3 (2020).
- Inventiva’s lanifibranor meets the primary and key secondary endpoints in the Phase IIb NATIVE clinical trial in non-alcoholic steatohepatitis (NASH). GlobeNewswire https://www.globenewswire.com/news-release/2020/06/15/2048284/0/en/Inventiva-s-lanifibranor-meets-the-primary-and-key-secondary-endpoints-in-the-Phase-IIb-NATIVE-clinical-trial-in-non-alcoholic-steatohepatitis-NASH.html (2020).
- Sayiner, M. et al. Variables Associated With Inpatient and Outpatient Resource Utilization Among Medicare Beneficiaries With Nonalcoholic Fatty Liver Disease With or Without Cirrhosis. J. Clin. Gastroenterol. (2017) doi:10.1097/MCG.0000000000000567.
- Madrigal Pharmaceuticals. Madrigal’s MGL-3196 Achieves Liver Biopsy Endpoints in Patients with Non-alcoholic Steatohepatitis (NASH) at 36 Weeks in Phase 2 Clinical Trial. Press Release https://ir.madrigalpharma.com/node/12791/pdf (2018).
- Harrison, S. A. et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (2019) doi:10.1016/S0140-6736(19)32517-6.
- Madrigal Pharmaceuticals. Madrigal MAESTRO Phase 3 NASH Trials Continue without Protocol Modifications; New Data Demonstrate that Reductions in Liver Fat Achieved by Resmetirom Predict NASH Resolution and Fibrosis Reduction. Press Release https://www.madrigalpharma.com/wp-content/uploads/2020/04/Madrigal.COVID-and-ABSTRACT-Press-release.FINAL_20200414.pdf (2020).
- Allergan to Acquire Tobira Therapeutics Expanding Global GI R&D Pipeline and Taking a Leading R&D Position in NASH. PR Newswire https://www.prnewswire.com/news-releases/allergan-to-acquire-tobira-therapeutics-expanding-global-gi-rd-pipeline-and-taking-a-leading-rd-position-in-nash-300330821.html (2016).
- New Data from CENTAUR Phase 2b Clinical Study Supports Continued Development of Cenicriviroc (CVC) in Ongoing Phase 3 AURORA Trial. PR Newswire https://www.prnewswire.com/news-releases/new-data-from-centaur-phase-2b-clinical-study-supports-continued-development-of-cenicriviroc-cvc-in-ongoing-phase-3-aurora-trial-300524237.html (2017).
- Novartis. Novartis expands development programs for NASH through clinical collaboration with Allergan. Press Release https://www.novartis.com/news/media-releases/novartis-expands-development-programs-nash-through-clinical-collaboration-allergan (2017).
- Akarna Therapeutics Ltd acquired by Allergan plc. Businesswire https://www.businesswire.com/news/home/20160920006848/en/Akarna-Therapeutics-acquired-Allergan-plc (2016).
- Galmed Pharmaceuticals. Phase 2 Data for Galmed Pharmaceutical’s Aramchol in Non-Alcoholic Steatohepatitis (NASH) Presented During Late-Breaking Abstract Oral Session of The Liver Meeting 2018. Press Release https://galmedpharma.investorroom.com/2018-11-13-Phase-2-Data-for-Galmed-Pharmaceuticals-Aramchol-TM-in-Non-Alcoholic-Steatohepatitis-NASH-Presented-During-Late-Breaking-Abstract-Oral-Session-of-The-Liver-Meeting-R-2018 (2018).
- Galmed Pharmaceuticals. Galmed Pharmaceuticals Unfolds the Development of Aramchol Non-Invasive Companion Diagnostic Program. Press Release https://galmedpharma.investorroom.com/2015-07-08-Galmed-Pharmaceuticals-Unfolds-the-Development-of-Aramchol-Non-Invasive-Companion-Diagnostic-Program (2015).
About The Authors:
 Mavra Nasir, Ph.D., consultant at Bay Life Science Advisors, supports strategic engagements across a range of therapeutic areas including rare diseases, hematology/oncology, and metabolic diseases for biopharma and medtech companies of all sizes. She has leveraged her scientific background and analytical expertise to help provide meaningful solutions to drug developers. Nasir joined Back Bay after receiving her Ph.D. in quantitative biomedical sciences from Dartmouth College. She has published research in non-invasive infectious disease diagnostics using advanced mass spectrometry and machine learning and presented at multiple national and international conferences. Nasir received her BSc in biological sciences from McGill University and MS in bioinformatics from New York University. Connect with her at info@bblsa.com.
Mavra Nasir, Ph.D., consultant at Bay Life Science Advisors, supports strategic engagements across a range of therapeutic areas including rare diseases, hematology/oncology, and metabolic diseases for biopharma and medtech companies of all sizes. She has leveraged her scientific background and analytical expertise to help provide meaningful solutions to drug developers. Nasir joined Back Bay after receiving her Ph.D. in quantitative biomedical sciences from Dartmouth College. She has published research in non-invasive infectious disease diagnostics using advanced mass spectrometry and machine learning and presented at multiple national and international conferences. Nasir received her BSc in biological sciences from McGill University and MS in bioinformatics from New York University. Connect with her at info@bblsa.com.
 Crystal Hsu, consultant at Back Bay Life Science Advisors, guides biotech, pharmaceutical, and medical device companies across an array of therapeutic areas. She has expertise in global pricing and market access, launch and go-to-market strategy, profit and revenue optimization, and landscape assessments for life science companies. Hsu has experience in evaluating market opportunities for life science companies in China. She graduated from Emory University’s Goizueta Business School with a BBA in finance and strategy & management consulting. Connect with her at info@bblsa.com.
Crystal Hsu, consultant at Back Bay Life Science Advisors, guides biotech, pharmaceutical, and medical device companies across an array of therapeutic areas. She has expertise in global pricing and market access, launch and go-to-market strategy, profit and revenue optimization, and landscape assessments for life science companies. Hsu has experience in evaluating market opportunities for life science companies in China. She graduated from Emory University’s Goizueta Business School with a BBA in finance and strategy & management consulting. Connect with her at info@bblsa.com.
 Peter Bak, Ph.D., SVP at Back Bay Life Science Advisors, has more than 10 years of experience with a broad range of research approaches — cellular, molecular and biochemical — and fields, from immunology and infection through oncology. He leads a diverse portfolio of projects with a focus on liquidity planning and positioning, strategic franchise building, M&A, and licensing strategy and buy-side diligence. Bak’s research career in immuno-oncology began at Dartmouth Medical School, where he received his Ph.D. in microbiology and immunology, and continued at MIT, as an American Cancer Society Postdoctoral Fellow at the Koch Institute of Integrative Cancer Research. He holds a BA in biology from The College of the Holy Cross. Connect with him at info@bblsa.com.
Peter Bak, Ph.D., SVP at Back Bay Life Science Advisors, has more than 10 years of experience with a broad range of research approaches — cellular, molecular and biochemical — and fields, from immunology and infection through oncology. He leads a diverse portfolio of projects with a focus on liquidity planning and positioning, strategic franchise building, M&A, and licensing strategy and buy-side diligence. Bak’s research career in immuno-oncology began at Dartmouth Medical School, where he received his Ph.D. in microbiology and immunology, and continued at MIT, as an American Cancer Society Postdoctoral Fellow at the Koch Institute of Integrative Cancer Research. He holds a BA in biology from The College of the Holy Cross. Connect with him at info@bblsa.com.
